
In chemistry, many authors consider an organic compound to be any chemical compound that contains carbon-hydrogen or carbon-carbon bonds, although the definition of "organic" versus "inorganic" varies from author to author, and is a topic of debate. For example, methane is considered organic, but whether some other carbon-containing compounds are organic or inorganic varies from author to author, for example halides of carbon without carbon-hydrogen bonds, and certain compounds of carbon with nitrogen and oxygen, which are generally considered inorganic.
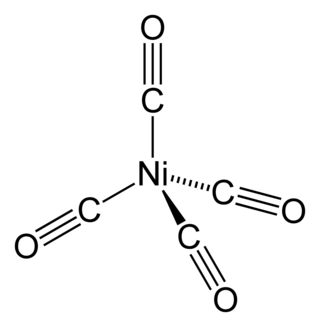
Nickel carbonyl (IUPAC name: tetracarbonylnickel) is a nickel(0) organometallic compound with the formula Ni(CO)4. This colorless liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for producing very high-purity nickel and a reagent in organometallic chemistry, although the Mond Process has fallen out of common usage due to the health hazards in working with the compound. Nickel carbonyl is one of the most dangerous substances yet encountered in nickel chemistry due to its very high toxicity, compounded with high volatility and rapid skin absorption.

Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.
The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemistry with Richard F. Heck and Ei-ichi Negishi for their contribution to the discovery and development of palladium-catalyzed cross-couplings in organic synthesis. This reaction is also known as the Suzuki–Miyaura reaction or simply as the Suzuki coupling. It is widely used to synthesize polyolefins, styrenes, and substituted biphenyls. Several reviews have been published describing advancements and the development of the Suzuki reaction. The general scheme for the Suzuki reaction is shown below, where a carbon-carbon single bond is formed by coupling a halide (R1-X) with an organoboron species (R2-BY2) using a palladium catalyst and a base.

Raney nickel, also called spongy nickel, is a fine-grained solid composed mostly of nickel derived from a nickel–aluminium alloy. Several grades are known, of which most are gray solids. Some are pyrophoric, but most are used as air-stable slurries. Raney nickel is used as a reagent and as a catalyst in organic chemistry. It was developed in 1926 by American engineer Murray Raney for the hydrogenation of vegetable oils. Raney is a registered trademark of W. R. Grace and Company. Other major producers are Evonik and Johnson Matthey.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.

2-Ethylhexanoic acid is the organic compound with the formula CH3(CH2)3CH(C2H5)CO2H. It is a carboxylic acid that is widely used to prepare lipophilic metal derivatives that are soluble in nonpolar organic solvents. 2-Ethylhexanoic acid is a colorless viscous oil. It is supplied as a racemic mixture.
The Mozingo reduction, also known as Mozingo reaction or thioketal reduction, is a chemical reaction capable of fully reducing a ketone or aldehyde to the corresponding alkane via a dithioacetal. The reaction scheme is as follows:
Nickel compounds are chemical compounds containing the element nickel which is a member of the group 10 of the periodic table. Most compounds in the group have an oxidation state of +2. Nickel is classified as a transition metal with nickel(II) having much chemical behaviour in common with iron(II) and cobalt(II). Many salts of nickel(II) are isomorphous with salts of magnesium due to the ionic radii of the cations being almost the same. Nickel forms many coordination complexes. Nickel tetracarbonyl was the first pure metal carbonyl produced, and is unusual in its volatility. Metalloproteins containing nickel are found in biological systems.
Lithium laurate is an metallorganic compound with the chemical formula LiO2C(CH2)10CH3. It is classified as a metallic soap, i.e. a metal derivative of a fatty acid. In contrast to the lubricants lithium stearate and lithium 12-hydroxystearate, lithium laurate is of minor commercial value..
Lead(II) laurate is an metal-organic compound with the chemical formula Pb(O2C 10CH3)2. It is classified as a metallic soap, i.e. a metal derivative of a fatty acid. Like most soaps, it does not dissolve in water. Lead soaps have been used as stabilizers and plasticizers in PVC.
Copper(II) laurate is an metal-organic compound with the chemical formula Cu(C
11H
23COO)
2. It is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Cobalt laurate is an metal-organic compound with the chemical formula C
24H
48CoO
4. It is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Zinc laurate is an metal-organic compound with the chemical formula C
24H
46ZnO
4. It is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Manganese laurate is an metal-organic compound with the chemical formula C
24H
48MnO
4. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Lanthanum laurate is an metal-organic compound with the chemical formula C
36H
72LaO
6. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Aluminium laurate is an metal-organic compound with the chemical formula C
36H
69AlO
6. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Magnesium laurate is a metal-organic compound with the chemical formula C
24H
46MgO
4. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Potassium laurate is a metal-organic compound with the chemical formula C
12H
23KO
2. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Nickel(II) stearate is a metal-organic compound, a salt of nickel and stearic acid with the chemical formula C
36H
70NiO
4. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid. The compound is harmful if swallowed and may cause skin sensitization.





