Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.

Nitric acid is the inorganic compound with the formula HNO3. It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% HNO3, it is referred to as fuming nitric acid. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%.

The nitronium ion, [NO2]+, is a cation. It is an onium ion because its nitrogen atom has +1 charge, similar to ammonium ion [NH4]+. It is created by the removal of an electron from the paramagnetic nitrogen dioxide molecule NO2, or the protonation of nitric acid HNO3.

An oxide is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– ion with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 that protects the foil from further oxidation.

Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide (NTO), and occasionally (usually among ex-USSR/Russian rocket engineers) as amyl, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is 92.011 g/mol.

Nitrogen dioxide is a chemical compound with the formula NO2. One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C2v point group symmetry. Industrially, NO2 is an intermediate in the synthesis of nitric acid, millions of tons of which are produced each year, primarily for the production of fertilizers.

Nitrous acid is a weak and monoprotic acid known only in solution, in the gas phase, and in the form of nitrite salts. It was discovered by Carl Wilhelm Scheele, who called it "phlogisticated acid of niter". Nitrous acid is used to make diazonium salts from amines. The resulting diazonium salts are reagents in azo coupling reactions to give azo dyes.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.
Dinitrogen oxide can potentially refer to any of at least four compounds:
In atmospheric chemistry, NOx is shorthand for nitric oxide and nitrogen dioxide, the nitrogen oxides that are most relevant for air pollution. These gases contribute to the formation of smog and acid rain, as well as affecting tropospheric ozone.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.

Reactive nitrogen species (RNS) are a family of antimicrobial molecules derived from nitric oxide (•NO) and superoxide (O2•−) produced via the enzymatic activity of inducible nitric oxide synthase 2 (NOS2) and NADPH oxidase respectively. NOS2 is expressed primarily in macrophages after induction by cytokines and microbial products, notably interferon-gamma (IFN-γ) and lipopolysaccharide (LPS).

Nitrogen trioxide or nitrate radical is an oxide of nitrogen with formula NO
3, consisting of three oxygen atoms covalently bound to a nitrogen atom. This highly unstable blue compound has not been isolated in pure form, but can be generated and observed as a short-lived component of gas, liquid, or solid systems.
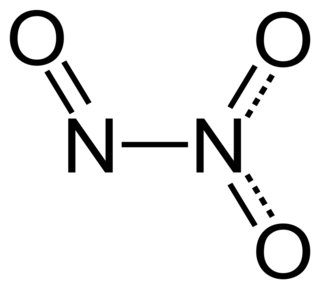
Dinitrogen trioxide is the inorganic compound with the formula N2O3. It is a nitrogen oxide. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21 °C (−6 °F):
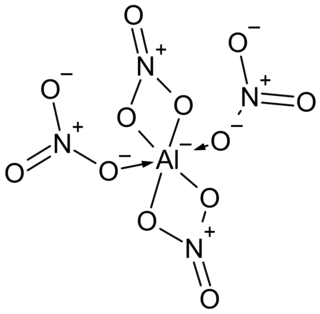
Tetranitratoaluminate is an anion of aluminium and nitrate groups with formula [Al(NO3)4]− that can form salts called tetranitratoaluminates. It is unusual in being a nitrate complex of a light element.

Titanium nitrate is the inorganic compound with formula Ti(NO3)4. It is a colorless, diamagnetic solid that sublimes readily. It is an unusual example of a volatile binary transition metal nitrate. Ill defined species called titanium nitrate are produced upon dissolution of titanium or its oxides in nitric acid.

Zirconium nitrate is a volatile anhydrous transition metal nitrate salt of zirconium with formula Zr(NO3)4. It has alternate names of zirconium tetranitrate, or zirconium(IV) nitrate.

Tin(IV) nitrate is a salt of tin with nitric acid. It is a volatile white solid, subliming at 40 °C under a vacuum. Unlike other nitrates, it reacts with water to produce nitrogen dioxide.

A transition metal nitrate complex is a coordination compound containing one or more nitrate ligands. Such complexes are common starting reagents for the preparation of other compounds.
Rhenium trioxynitrate, also known as rhenium(VII) trioxide nitrate, is a chemical compound with the formula ReO3NO3. It is a white solid that readily hydrolyzes in moist air.