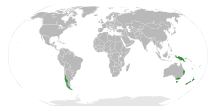
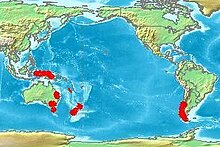
Araucaria is a genus of evergreen coniferous trees in the family Araucariaceae. While today they are largely confined to the Southern Hemisphere, during the Jurassic and Cretaceous they were distributed globally. There are 20 extant species in New Caledonia, Norfolk Island, eastern Australia, New Guinea, Argentina, Brazil and Chile.

Antarctic flora are a distinct community of vascular plants which evolved millions of years ago on the supercontinent of Gondwana. Presently, species of Antarctica flora reside on several now separated areas of the Southern Hemisphere, including southern South America, southernmost Africa, New Zealand, Australia, and New Caledonia. Joseph Dalton Hooker was the first to notice similarities in the flora and speculated that Antarctica had served as either a source or a transitional point, and that land masses now separated might formerly have been adjacent.

The Atherospermataceae, commonly known as the southern sassafrases, are a family of broadleaf evergreen trees and shrubs. The family includes 14 species in seven genera. The atherosperms are today mostly distributed in the Southern Hemisphere, with two species native to southern Chile and 12 species native to Australasia. Wood is commercially harvested from rainforest species of this family, and is used both in construction and in fine cabinet making.

The biodiversity of New Caledonia is of exceptional biological and paleoecological interest. It is frequently referred to as a biodiversity hotspot. The country is a large South Pacific archipelago with a total land area of more than 18,000 square kilometres (6,900 sq mi). The terrain includes a variety of reefs, atolls, small islands, and a variety of topographical and edaphic regions on the largest island, all of which promote the development of unusually concentrated biodiversity. The region's climate is oceanic and tropical.

The New Caledonia rain forests are a terrestrial ecoregion, located in New Caledonia in the South Pacific. It is a tropical moist broadleaf forest ecoregion, part of the Australasian realm.

Nothofagus gunnii, the tanglefoot or deciduous beech, is a deciduous shrub or small tree endemic to the highlands of Tasmania, Australia. It was described in 1847 by R.C Gunn N. gunnii is a small woody tree with a shrubby appearance known to grow up to 8 metres (26 ft). It lives only on mountains due to temperature limitations within the Tasmanian maritime climate and mainly grows at altitudes greater than 800 metres (2,600 ft) above sea level. It grows in alpine and sub-alpine regions in the central portions of the island. Though capable of reaching the size of a small tree, it is most common as a thick shrub or woody ground cover, hence its common name of "tanglefoot".

Nothofagus moorei, commonly known as Antarctic beech, is an important Gondwana relict of the rainforests of the southern hemisphere. It occurs in wet, fire-free areas at high altitude in eastern Australia.

Nothofagus glauca, commonly known as hualo or roble Maulino, is a species of plant in the family Nothofagaceae. It is a deciduous tree endemic to Chile. It grows from 34° to 37° South latitude. It is a typical tree of the maritime mediterranean-climate Maulino forest of Central Chile, its current range spanning over 330 km from north to south. The species grows on a variety of soils and is mostly found on gentle to steep slopes.
Nothofagus nuda is a species of plant in the family Nothofagaceae. It is endemic to Papua New Guinea. It is threatened by habitat loss.
Nothofagus stylosa is a species of plant in the family Nothofagaceae. It is endemic to West Papua (Indonesia). It is a Critically Endangered species threatened by habitat loss.

The Paleotropical Kingdom (Paleotropis) is a floristic kingdom composed of the tropical areas of Africa, Asia and Oceania, as proposed by Ronald Good and Armen Takhtajan. Part of its flora is inherited from the ancient supercontinent of Gondwana or exchanged later. These Gondwanan lineages are related to those in the Neotropical Kingdom, composed of the tropical areas of Central and South America. Flora from the Paleotropical Kingdom influenced the tropical flora of the Australian Kingdom. The kingdom is subdivided into five floristic subkingdoms according to Takhtajan and about 13 floristic regions. In this article the floristic subkingdoms and regions are given as delineated by Takhtajan.
The natural history of New Zealand began when the landmass Zealandia broke away from the supercontinent Gondwana in the Cretaceous period. Before this time, Zealandia shared its past with Australia and Antarctica. Since this separation, the New Zealand landscape has evolved in physical isolation, although much of its current biota has more recent connections with species on other landmasses. The exclusively natural history of the country ended in about 1300 AD, when humans first settled, and the country's environmental history began. The period from 1300 AD to today coincides with the extinction of many of New Zealand's unique species that had evolved there.

Gondwana was a large landmass, sometimes referred to as a supercontinent. The remnants of Gondwana make up around two-thirds of today's continental area, including South America, Africa, Antarctica, Australia, Zealandia, Arabia, and the Indian Subcontinent.

The biodiversity of Tasmania is of exceptional biological and paleoecological interest. A state of Australia, it is a large South Pacific archipelago of one large main island and a range of smaller islands. The terrain includes a variety of reefs, atolls, many small islands, and a variety of topographical and edaphic regions on the largest island, all of which promote the development of unusually concentrated biodiversity. During long periods geographically and genetically isolated, it is known for its unique flora and fauna. The region's climate is oceanic.

Nothofagus cliffortioides, commonly called mountain beech, is a species of Southern beech tree and is endemic to New Zealand. Mountain beech grows in mountainous regions at high elevations. In New Zealand the taxon is called Fuscospora cliffortioides. Nothofagus cliffortioides occupies a wider range of habitat than any other New Zealand tree species and it shows a corresponding range of life form, seeding habits, regenerative patterns, growth habits, growth rates, stand replacement and mortality patterns.
Jansonius is a genus of leaf beetles in the subfamily Eumolpinae. It is found in Chile and Argentina. It was formerly placed in the tribe Adoxini, section Myochroites, but is now placed in Nodinini, section Metachromites.
Nothofagus brassii is a species of tree in the family Nothofagaceae. It is endemic to New Guinea. It is commonly known as Sagé, sagé hitam, sahé, and kayu sagé, kayu sagé hitam (Indonesian).
Nothofagus crenata is a species of tree in the family Nothofagaceae. It is endemic to New Guinea. It grows in lowland rain forest below 900 meters elevation.
Nothofagus starkenborghiorum is a species of tree in the family Nothofagaceae. It is native to New Guinea and New Britain. It grows in montane rain forests, and occasionally in lowland rain forests.