Related Research Articles
Daclizumab is a therapeutic humanized monoclonal antibody which was used for the treatment of adults with relapsing forms of multiple sclerosis (MS). Daclizumab works by binding to CD25, the alpha subunit of the IL-2 receptor of T-cells.

Rituximab, sold under the brand name Rituxan among others, is a monoclonal antibody medication used to treat certain autoimmune diseases and types of cancer. It is used for non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, granulomatosis with polyangiitis, idiopathic thrombocytopenic purpura, pemphigus vulgaris, myasthenia gravis and Epstein–Barr virus-positive mucocutaneous ulcers. It is given by slow injection into a vein. Biosimilars of Rituxan include Blitzima, Riabni, Ritemvia, Rituenza, Rixathon, Ruxience, and Truxima.

Cancer immunotherapy is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.

Ibritumomab tiuxetan, sold under the trade name Zevalin, is a monoclonal antibody radioimmunotherapy treatment for non-Hodgkin's lymphoma. The drug uses the monoclonal mouse IgG1 antibody ibritumomab in conjunction with the chelator tiuxetan, to which a radioactive isotope is added. Tiuxetan is a modified version of DTPA whose carbon backbone contains an isothiocyanatobenzyl and a methyl group.
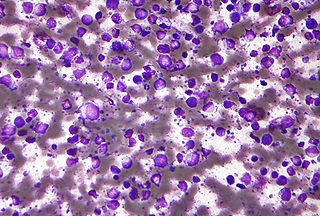
Diffuse large B-cell lymphoma (DLBCL) is a cancer of B cells, a type of lymphocyte that is responsible for producing antibodies. It is the most common form of non-Hodgkin lymphoma among adults, with an annual incidence of 7–8 cases per 100,000 people per year in the US and UK. This cancer occurs primarily in older individuals, with a median age of diagnosis at ~70 years, although it can occur in young adults and, in rare cases, children. DLBCL can arise in virtually any part of the body and, depending on various factors, is often a very aggressive malignancy. The first sign of this illness is typically the observation of a rapidly growing mass or tissue infiltration that is sometimes associated with systemic B symptoms, e.g. fever, weight loss, and night sweats.

Monoclonal antibody therapy is a form of immunotherapy that uses monoclonal antibodies (mAbs) to bind monospecifically to certain cells or proteins. The objective is that this treatment will stimulate the patient's immune system to attack those cells. Alternatively, in radioimmunotherapy a radioactive dose localizes a target cell line, delivering lethal chemical doses. Antibodies have been used to bind to molecules involved in T-cell regulation to remove inhibitory pathways that block T-cell responses. This is known as immune checkpoint therapy.
Toralizumab was a humanized monoclonal antibody and an immunosuppressive drug. Possible indications included treatment of antibody-mediated disorders, T-cell-mediated diseases, and B-cell malignancies such as CLL/small lymphocytic lymphoma, follicular cell lymphoma grade I or II, marginal zone lymphoma, mantle cell lymphoma, MALT lymphoma, Waldenström's macroglobulinemia, monocytoid B-cell lymphoma; relapsed/refractory Hodgkin's disease).
Lintuzumab (SGN-33) is a humanized monoclonal antibody used in the treatment of cancer. The drug had been developed by Seattle Genetics as a treatment for acute myeloid leukemia (AML), a condition which results in the deaths of 9,000 people a year in the United States. Lintuzumab targets the CD33 protein, which is expressed in AML and other myeloproliferative diseases, but does not appear in abundance on normal cells.
Epratuzumab is a humanized monoclonal antibody. Potential uses may be found in oncology and in treatment of inflammatory autoimmune disorders, such as systemic lupus erythematosus (SLE).
Ofatumumab is a fully human monoclonal antibody to CD20, which appears to provide rapid B-cell depletion. Under the brand name Kesimpta, it is approved for the treatment of multiple sclerosis in the United States as well as in the European Union and other regions. Under the brand name Arzerra, it is approved for the treatment of certain types of chronic lymphocytic leukemia (CLL) in the United States. It is sold by Novartis under license from Genmab.
CD16, also known as FcγRIII, is a cluster of differentiation molecule found on the surface of natural killer cells, neutrophils, monocytes, macrophages, and certain T cells. CD16 has been identified as Fc receptors FcγRIIIa (CD16a) and FcγRIIIb (CD16b), which participate in signal transduction. The most well-researched membrane receptor implicated in triggering lysis by NK cells, CD16 is a molecule of the immunoglobulin superfamily (IgSF) involved in antibody-dependent cellular cytotoxicity (ADCC). It can be used to isolate populations of specific immune cells through fluorescent-activated cell sorting (FACS) or magnetic-activated cell sorting, using antibodies directed towards CD16.
An anti-CD22 immunotoxin is a monoclonal antibody linked to a cytotoxic agent. They are being studied in the treatment of some types of B-cell cancer.
Genmab A/S is a Danish biotechnology company, founded in February 1999 by Florian Schönharting, at the time managing director of BankInvest Biomedical venture fund. The company is based in Copenhagen, Denmark – internationally, it operates through the subsidiaries Genmab B.V. in Utrecht, The Netherlands, Genmab U.S., Inc. in Princeton, USA, and Genmab K.K. in Tokyo, Japan. It's a dual listed company with shares traded on the Copenhagen Stock Exchange in Denmark, and on NASDAQ Global Select Market in the US.
Obinutuzumab, sold under the brand name Gazyva among others, is a humanized anti-CD20 monoclonal antibody, originated by GlycArt Biotechnology AG and developed by Roche as a cancer treatment.
Atacicept is a recombinant fusion protein designed to inhibit B cells, thereby suppressing autoimmune disease. The designer protein combines the binding site for two cytokines that regulate maturation, function, and survival of B cells - B-lymphocyte stimulator (BLyS) and A proliferation-inducing ligand (APRIL), with the constant region of immunoglobin. Atacicept blocks activation of B cells by the tumor necrosis factor receptor superfamily member 13B, a transmembrane receptor protein found predominantly on the surface of B cells. Like the monoclonal antibody belimumab, atacicept blocks the binding of BLyS, but it also blocks APRIL. Binding of these TACI ligands induces proliferation, activation, and longevity of B cells and thus their production of autoantibodies. Atacicept is thought to selectively impair mature B cells and plasma cells with less impact on progenitor cells and memory B cells.
Gene expression profiling has revealed that diffuse large B-cell lymphoma (DLBCL) is composed of at least 3 different sub-groups, each having distinct oncogenic mechanisms that respond to therapies in different ways. Germinal Center B-Cell like (GCB) DLBCLs appear to arise from normal germinal center B cells, while Activated B-cell like (ABC) DLBCLs are thought to arise from postgerminal center B cells that are arrested during plasmacytic differentiation. The differences in gene expression between GCB DLBCL and ABC DLBCL are as vast as the differences between distinct types of leukemia, but these conditions have historically been grouped together and treated as the same disease.
Urelumab is a fully human, non‐ligand binding, CD137 agonist immunoglobulin‐γ 4 (IgG4) monoclonal antibody. It was developed utilizing Medarex's UltiMAb(R) technology by Bristol-Myers Squibb for the treatment of cancer and solid tumors. Urelumab promotes anti-tumor immunity, or an immune response against tumor cells, via CD137 activation. The application of Urelumab has been limited due to the fact that it can cause severe liver toxicity.

Daratumumab, sold under the brand name Darzalex, is an anti-cancer monoclonal antibody medication. It binds to CD38, which is overexpressed in multiple myeloma cells. Daratumumab was originally developed by Genmab, but it is now being jointly developed by Genmab along with the Johnson & Johnson subsidiary Janssen Biotech, which acquired worldwide commercialization rights to the drug from Genmab.
Pidilizumab is a monoclonal antibody being developed by Medivation for the treatment of cancer and infectious diseases. Pidilizumab was originally thought to bind to the PD-1 immune checkpoint molecule, however, recent evidence suggests that Delta-like 1 (DLL1) is its primary binding target while binding to PD-1 is secondary and restricted to non-glycosylated and hypoglycosylated forms of this molecule. Pidilizumab causes in the attenuation of apoptotic processes in lymphocytes, primarily effector/memory T cells.
Seagen Inc. is an American biotechnology company focused on developing and commercializing innovative, empowered monoclonal antibody-based therapies for the treatment of cancer. The company, headquartered in Bothell, Washington, is the industry leader in antibody-drug conjugates or ADCs, a technology designed to harness the targeting ability of monoclonal antibodies to deliver cell-killing agents directly to cancer cells. Antibody-drug conjugates are intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy, while potentially enhancing antitumor activity.
References
- ↑ "Statement On A Nonproprietary Name Adopted By The USAN Council - Ocaratuzumab" (PDF). American Medical Association.
- 1 2 3 4 5 Forero-Torres A, de Vos S, Pohlman BL, Pashkevich M, Cronier DM, Dang NH, et al. (March 2012). "Results of a phase 1 study of AME-133v (LY2469298), an Fc-engineered humanized monoclonal anti-CD20 antibody, in FcγRIIIa-genotyped patients with previously treated follicular lymphoma". Clinical Cancer Research. 18 (5): 1395–403. doi: 10.1158/1078-0432.CCR-11-0850 . PMID 22223529.
- 1 2 3 "mentrik.com".
- 1 2 3 Tobinai K, Ogura M, Kobayashi Y, Uchida T, Watanabe T, Oyama T, et al. (February 2011). "Phase I study of LY2469298, an Fc-engineered humanized anti-CD20 antibody, in patients with relapsed or refractory follicular lymphoma". Cancer Science. 102 (2): 432–8. doi:10.1111/j.1349-7006.2010.01809.x. PMID 21205069. S2CID 26346792.