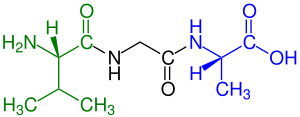Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.

Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthesis is most commonly performed by ribosomes in cells. Peptides can also be synthesized in the laboratory. Protein primary structures can be directly sequenced, or inferred from DNA sequences.

α-Amanitin (alpha-Amanitin) is a cyclic peptide of eight amino acids. It is possibly the most deadly of all the amatoxins, toxins found in several species of the mushroom genus Amanita, one being the death cap as well as the destroying angel, a complex of similar species, principally A. virosa and A. bisporigera. It is also found in the mushrooms Galerina marginata and Conocybe filaris. The oral LD50 of amanitin is 100 μg/kg for rats.

A tripeptide is a peptide derived from three amino acids joined by two or sometimes three peptide bonds. As for proteins, the function of peptides is determined by the constituent amino acids and their sequence. In terms of scientific investigations, the dominant tripeptide is glutathione (γ-L-Glutamyl-L-cysteinylglycine), which serves many roles in many forms of life.

Microcystins—or cyanoginosins—are a class of toxins produced by certain freshwater cyanobacteria, commonly known as blue-green algae. Over 250 different microcystins have been discovered so far, of which microcystin-LR is the most common. Chemically they are cyclic heptapeptides produced through nonribosomal peptide synthases.

Amanita virosa is a species of fungus in the class Agaricomycetes. In the UK, it has the recommended English name of destroying angel and is known internationally as the European destroying angel. Basidiocarps are agaricoid (mushroom-shaped) and pure white with a ring on the stem and a sack-like volva at the base. The species is deadly poisonous. It occurs in Europe and northern Asia. Amanita virosa was formerly reported from North America, but research has shown that similar-looking American species, including Amanita bisporigera and A. ocreata, are distinct.
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term nonribosomal peptide typically refers to a very specific set of these as discussed in this article.
Amatoxin is the collective name of a subgroup of at least nine related toxic compounds found in three genera of poisonous mushrooms and one species of the genus Pholiotina. Amatoxins are very potent, as little as half a mushroom cap can cause severe liver injury if swallowed.

Cyclic peptides are polypeptide chains which contain a circular sequence of bonds. This can be through a connection between the amino and carboxyl ends of the peptide, for example in cyclosporin; a connection between the amino end and a side chain, for example in bacitracin; the carboxyl end and a side chain, for example in colistin; or two side chains or more complicated arrangements, for example in alpha-amanitin. Many cyclic peptides have been discovered in nature and many others have been synthesized in the laboratory. Their length ranges from just two amino acid residues to hundreds. In nature they are frequently antimicrobial or toxic; in medicine they have various applications, for example as antibiotics and immunosuppressive agents. Thin-Layer Chromatography (TLC) is a convenient method to detect cyclic peptides in crude extract from bio-mass.
A tetrapeptide is a peptide, classified as an oligopeptide, since it only consists of four amino acids joined by peptide bonds. Many tetrapeptides are pharmacologically active, often showing affinity and specificity for a variety of receptors in protein-protein signaling. Present in nature are both linear and cyclic tetrapeptides (CTPs), the latter of which mimics protein reverse turns which are often present on the surface of proteins and druggable targets. Tetrapeptides may be cyclized by a fourth peptide bond or other covalent bonds.

Calciseptine (CaS) is a natural neurotoxin isolated from the black mamba Dendroaspis p. polylepis venom. This toxin consists of 60 amino acids with four disulfide bonds. Calciseptine specifically blocks L-type calcium channels, but not other voltage-dependent Ca2+ channels such as N-type and T-type channels.
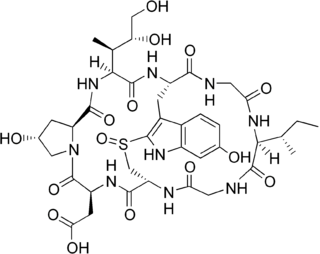
β-Amanitin (beta-Amanitin) is a cyclic peptide comprising eight amino acids. It is part of a group of toxins called amatoxins, which can be found in several mushrooms belonging to the genus Amanita. Some examples are the death cap and members of the destroying angel complex, which includes A. virosa and A. bisporigera. Due to the presence of α-Amanitin, β-Amanitin, γ-Amanitin and epsilon-Amanitin these mushrooms are highly lethal to human beings.
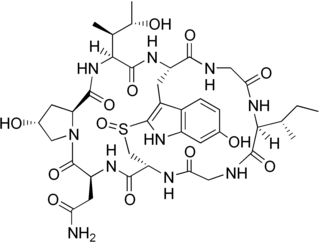
γ-Amanitin (gamma-Amanitin) is a cyclic peptide of eight amino acids. It is an amatoxin, a group of toxins isolated from and found in several members of the mushroom genus Amanita, one being the death cap as well as the destroying angel, a complex of similar species, principally A. virosa and A. bisporigera. The compound is highly toxic, inhibits RNA polymerase II, disrupts synthesis of mRNA, and can be fatal.
Phallolysin is a protein found the Amanita phalloides species of the Amanita genus of mushrooms, the species commonly known as the death cap mushroom. The protein is toxic and causes cytolysis in many cells found in animals and is noted for its hemolytic properties. It was one of the first toxins discovered in Amanita phalloides when the various toxins in the species where first being researched. The protein itself is observed to come in 3 variations, with observed differences in isoelectric point. Cytolysis can be best described as being the destruction of cells, likely due to exposure from an external source such as pathogens and toxins. Hemolysis then follows a similar destructive pathway, but instead focuses specifically on the destruction of red blood cells. Phallolysin is known to be thermolabile, meaning that it is destroyed at high temperatures, and acid labile, meaning that it is easily broken down in acidic environments.

Antamanide is a cyclic decapeptide isolated from a fungus, the death cap: Amanita phalloides. It is being studied as a potential anti-toxin against the effects of phalloidin and for its potential for treating edema. It contains 1 valine residue, 4 proline residues, 1 alanine residue, and 4 phenylalanine residues with a structure of c(Val-Pro-Pro-Ala-Phe-Phe-Pro-Pro-Phe-Phe). It was isolated by determining the source of the anti-phalloidin activity from a lipophillic extraction from the organism. It has been shown that antamanide can react to form alkali metal ion complexes. These include complexes with sodium and calcium ions. When these complexes are formed, the cyclopeptide structure undergoes a conformational change.

Microcystin-LR (MC-LR) is a toxin produced by cyanobacteria. It is the most toxic of the microcystins.

Amanita exitialis, also known as the Guangzhou destroying angel, is a mushroom of the large genus Amanita. It is distributed in eastern Asia, and probably also in India where it has been misidentified as A. verna. Deadly poisonous, it is a member of section Phalloideae and related to the death cap A. phalloides. The fruit bodies (mushrooms) are white, small to medium-sized with caps up to 7 cm (2.8 in) in diameter, a somewhat friable ring and a firm volva. Unlike most agaric mushrooms which typically have four-spored basidia, the basidia of A. exitialis are almost entirely two-spored. Eight people were fatally poisoned in China after consuming the mushroom in 2000, and another 20 have been fatally poisoned since that incident. Molecular analysis shows that the species has a close phylogenetic relationship with three other toxic white Amanitas: A. subjunquillea var. alba, A. virosa and A. bisporigera.

Amanita sphaerobulbosa, commonly known as the Asian abrupt-bulbed Lepidella, is a species of agaric fungus in the family Amanitaceae. First described by mycologist Tsuguo Hongo in 1969, it is found in Southern Asia.
Cyanopeptolins (CPs) are a class of oligopeptides produced by Microcystis and Planktothrix algae strains, and can be neurotoxic. The production of cyanopeptolins occurs through nonribosomal peptides synthases (NRPS).