
An insulin pump is a medical device used for the administration of insulin in the treatment of diabetes mellitus, also known as continuous subcutaneous insulin therapy. The device configuration may vary depending on design. A traditional pump includes:

Medtronic plc is an American medical device company. The company's operational and executive headquarters are in Minneapolis, Minnesota, and its legal headquarters are in Ireland due to its acquisition of Irish-based Covidien in 2015. While it primarily operates in the United States, it operates in more than 150 countries and employs over 90,000 people. It develops and manufactures healthcare technologies and therapies.
JDRF is a nonprofit 501(c)(3) organization that funds type 1 diabetes (T1D) research, provides a broad array of community and activist services to the T1D population and actively advocates for regulation favorable to medical research and approval of new and improved treatment modalities. It was initially founded as the JDF, the Juvenile Diabetes Foundation. It later changed its name to the Juvenile Diabetes Research Foundation and is now known as JDRF.
Automated insulin delivery systems are automated systems designed to assist people with insulin-requiring diabetes, by automatically adjusting insulin delivery in response to blood glucose levels. Currently available systems can only deliver a single hormone—insulin. Other systems currently in development aim to improve on current systems by adding one or more additional hormones that can be delivered as needed, providing something closer to the endocrine functionality of the pancreas.
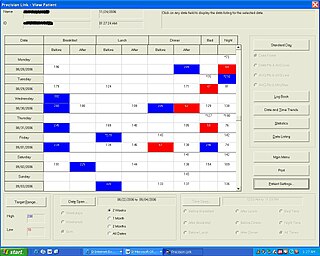
Diabetes Management Software refers to software tools that run on personal computers and personal digital assistants to help persons with Type 1 and Type 2 diabetes manage the data associated with:
Animas Corporation was an American company that specialized in making insulin pumps. The company was founded by Katherine Crothall in 1996, had its initial public offering in May 2004 under the ticker symbol 'PUMP', and was ultimately acquired by Johnson & Johnson on February 18, 2006. The business was headquartered in West Chester, Pennsylvania and forms part of the Johnson & Johnson Diabetes franchise along with Lifescan and several other companies producing medical products for the treatment and management of diabetes.
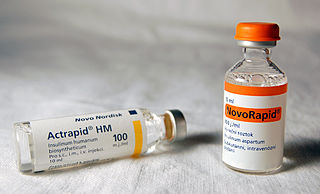
As a medication, insulin is any pharmaceutical preparation of the protein hormone insulin that is used to treat high blood glucose. Such conditions include type 1 diabetes, type 2 diabetes, gestational diabetes, and complications of diabetes such as diabetic ketoacidosis and hyperosmolar hyperglycemic states. Insulin is also used along with glucose to treat hyperkalemia. Typically it is given by injection under the skin, but some forms may also be used by injection into a vein or muscle. There are various types of insulin, suitable for various time spans. The types are often all called insulin in the broad sense, although in a more precise sense, insulin is identical to the naturally occurring molecule whereas insulin analogues have slightly different molecules that allow for modified time of action. It is on the World Health Organization's List of Essential Medicines. In 2020, regular human insulin was the 307th most commonly prescribed medication in the United States, with more than 1 million prescriptions.

MiniMed Paradigm is a series of insulin pumps manufactured by Medtronic for patients with diabetes mellitus. The pump operates with a single AAA battery and uses a piston-plunger pump to infuse a programmed amount of insulin into the patient through a length of tubing. The Paradigm uses a one-way wireless radio frequency link to receive blood sugar measurements from select glucose meters. The Paradigm RT series adds the ability to receive data from a mated continuous blood-glucose monitor. Although the pump can use these measurements to assist in calculating a dose of insulin, no actual change in insulin delivery occurs without manual user-intervention.
DexCom, Inc. is a company that develops, manufactures, produces, and distributes continuous glucose monitoring (CGM) systems for diabetes management. It operates internationally with headquarters in San Diego, California, and has manufacturing facilities in Mesa, Arizona and Batu Kawan, Malaysia.
Glucommander is a computer-directed method of inpatient glucose management.
International Diabetes Center at Park Nicollet (IDC) is a center for diabetes care, research and education located in Minneapolis, Minnesota, United States. The center provides clinical, motivational and educational services for people with diabetes. It is part of HealthPartners Institute.
Tandem Diabetes Care is an American medical device manufacturer based in San Diego, California. The company develops medical technologies for the treatment of diabetes and specifically insulin infusion therapy.
Ambulatory glucose profile (AGP) is a single-page, standardized report for interpreting a patient's daily glucose and insulin patterns. AGP provides both graphic and quantitative characterizations of daily glucose patterns. First developed by Drs. Roger Mazze and David Rodbard, with colleagues at the Albert Einstein College of Medicine in 1987, AGP was initially used for the representation of episodic self-monitored blood glucose (SMBG). The first version included a glucose median and inter-quartile ranges graphed as a 24-hour day. Dr. Mazze brought the original AGP to the International Diabetes Center (IDC) in the late 1980s. Since then, IDC has built the AGP into the internationally recognized standard for glucose pattern reporting.
Bigfoot Biomedical Inc. is a medical technology start-up headquartered in Milpitas, California, founded by a team of people with personal connections to type 1 and type 2 diabetes.
Nightscout is a free and open-source project, and associated social movement, that enables accessing and working with continuous glucose monitor (CGM) data. Nightscout software aims to give users access to their real time blood sugar data by putting this data in the cloud. In addition to browser-based data visualization, Nightscout can also be used to review data from a phone or smartwatch, or to remotely monitor CGM data for individuals with type 1 diabetes. Associated with Nightscout software is a broader "CGM in the Cloud" social movement, supporting individuals seeking to access and use realtime CGM data through commercial and DIY approaches.

A continuous glucose monitor (CGM) is a device used for monitoring blood glucose on a continual basis by insulin-requiring people with diabetes, e.g. people with type I, type II diabetes or other types of diabetes. A continuous glucose monitor consists of three parts: a small electrode placed under the skin, a transmitter sending readings at regular intervals, and a separate receiver. Currently approved CGMs use an enzymatic technology which reacts with glucose molecules in the interstitial fluid generating an electric current. This electric current is then relayed from a transmitter attached to the sensor out to a reader which displays the data to the patient.

The Open Insulin Project is a community of researchers and advocates working to develop an open-source protocol for producing insulin that is affordable, has transparent pricing, and is community-owned.
Tidepool is a nonprofit company founded in 2013 which makes open-source tools to help people better manage diabetes. The company works together with Medtronic to create an interoperable automated insulin pump system.
Robin Koops is a Dutch mechanical engineer, designer and inventor. He is known for developing an artificial pancreas.







