
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.

Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike an ordinary metallic conductor, whose resistance decreases gradually as its temperature is lowered, even down to near absolute zero, a superconductor has a characteristic critical temperature below which the resistance drops abruptly to zero. An electric current through a loop of superconducting wire can persist indefinitely with no power source.

In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many intermediate states are known to exist, such as liquid crystal, and some states only exist under extreme conditions, such as Bose–Einstein condensates, neutron-degenerate matter, and quark–gluon plasma. For a complete list of all exotic states of matter, see the list of states of matter.
Unconventional superconductors are materials that display superconductivity which does not conform to conventional BCS theory or its extensions.

High-temperature superconductors are defined as materials with critical temperature above 77 K, the boiling point of liquid nitrogen. They are only "high-temperature" relative to previously known superconductors, which function at even colder temperatures, close to absolute zero. The "high temperatures" are still far below ambient, and therefore require cooling. The first break through of high-temperature superconductor was discovered in 1986 by IBM researchers Georg Bednorz and K. Alex Müller. Although the critical temperature is around 35.1 K, this new type of superconductor was readily modified by Ching-Wu Chu to make the first high-temperature superconductor with critical temperature 93 K. Bednorz and Müller were awarded the Nobel Prize in Physics in 1987 "for their important break-through in the discovery of superconductivity in ceramic materials". Most high-Tc materials are type-II superconductors.
A room-temperature superconductor is a material capable of displaying superconductivity at temperatures above 0 °C, which are commonly encountered in everyday settings. As of 2023, the material with the highest accepted superconducting temperature was highly pressurized lanthanum decahydride, whose transition temperature is approximately 250 K (−23 °C) at 200 GPa.

In chemistry, catenation is the bonding of atoms of the same element into a series, called a chain. A chain or a ring shape may be open if its ends are not bonded to each other, or closed if they are bonded in a ring. The words to catenate and catenation reflect the Latin root catena, "chain".

In chemistry, a charge-transfer (CT) complex or electron-donor-acceptor complex describes a type of supramolecular assembly of two or more molecules or ions. The assembly consists of two molecules that self-attract through electrostatic forces, i.e., one has at least partial negative charge and the partner has partial positive charge, referred to respectively as the electron acceptor and electron donor. In some cases, the degree of charge transfer is "complete", such that the CT complex can be classified as a salt. In other cases, the charge-transfer association is weak, and the interaction can be disrupted easily by polar solvents.

In chemistry, cryptands are a family of synthetic, bicyclic and polycyclic, multidentate ligands for a variety of cations. The Nobel Prize for Chemistry in 1987 was given to Donald J. Cram, Jean-Marie Lehn, and Charles J. Pedersen for their efforts in discovering and determining uses of cryptands and crown ethers, thus launching the now flourishing field of supramolecular chemistry. The term cryptand implies that this ligand binds substrates in a crypt, interring the guest as in a burial. These molecules are three-dimensional analogues of crown ethers but are more selective and strong as complexes for the guest ions. The resulting complexes are lipophilic.
An electride is an ionic compound in which an electron is the anion. Solutions of alkali metals in ammonia are electride salts. In the case of sodium, these blue solutions consist of [Na(NH3)6]+ and solvated electrons:

In organic chemistry, a Bechgaard salt is any one of a number of organic charge-transfer complexes that exhibit superconductivity at low temperatures. They are named for chemist Klaus Bechgaard, who was one of the first scientists to synthesize them and demonstrate their superconductivity with the help of physicist Denis Jérome. Most Bechgaard salt superconductors are extremely low temperature, and lose superconductivity above the 1–2 K range, although the most successful compound in this class superconducts up to almost 12 K.

Tetrathiafulvalene (TTF) is an organosulfur compound with the formula 2. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, (C5H4)2, by replacement of four CH groups with sulfur atoms. Over 10,000 scientific publications discuss TTF and its derivatives.
Charge ordering (CO) is a phase transition occurring mostly in strongly correlated materials such as transition metal oxides or organic conductors. Due to the strong interaction between electrons, charges are localized on different sites leading to a disproportionation and an ordered superlattice. It appears in different patterns ranging from vertical to horizontal stripes to a checkerboard–like pattern , and it is not limited to the two-dimensional case. The charge order transition is accompanied by symmetry breaking and may lead to ferroelectricity. It is often found in close proximity to superconductivity and colossal magnetoresistance.
The Fulde–Ferrell–Larkin–Ovchinnikov (FFLO) phase can arise in a superconductor in large magnetic field. Among its characteristics are Cooper pairs with nonzero total momentum and a spatially non-uniform order parameter, leading to normal conducting areas in the superconductor.
Antiperovskites is a type of crystal structure similar to the perovskite structure that is common in nature. The key difference is that the positions of the cation and anion constituents are reversed in the unit cell structure. In contrast to perovskite, antiperovskite compounds consist of two types of anions coordinated with one type of cation. Antiperovskite compounds are an important class of materials because they exhibit interesting and useful physical properties not found in perovskite materials, including as electrolytes in solid-state batteries.

Dicyanamide, also known as dicyanamine, is an anion having the formula C
2N−
3. It contains two cyanide groups bound to a central nitrogen anion. The chemical is formed by decomposition of 2-cyanoguanidine. It is used extensively as a counterion of organic and inorganic salts, and also as a reactant for the synthesis of various covalent organic structures.
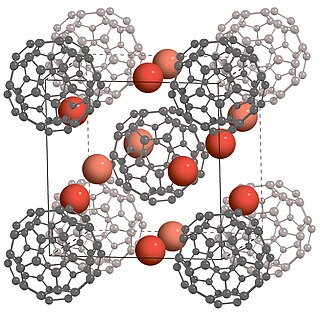
Fullerides are chemical compounds containing fullerene anions. Common fullerides are derivatives of the most common fullerenes, i.e. C60 and C70. The scope of the area is large because multiple charges are possible, i.e., [C60]n− (n = 1, 2...6), and all fullerenes can be converted to fullerides. The suffix "-ide" implies their negatively charged nature.
Lanthanum decahydride is a polyhydride or superhydride compound of lanthanum and hydrogen (LaH10) that has shown evidence of being a high-temperature superconductor. It has a superconducting transition temperature TC around 250 K (−23 °C; −10 °F) at a pressure of 150 gigapascals (GPa), and its synthesis required pressures above ~160 GPa.

Denis Jerome is a French experimental physicist in the field of condensed matter, who contributed to the discovery of superconductivity in organic conductive matter.
Conduction zone refers to the network of electron paths between molecules in a conductor where electrons can flow along the paths at the same energy level, resulting in currents. An electron, with an energy level below the conduction zone, remains confined within its orbital inside the individual molecule and it cannot produce any current. To create currents in a conductor, electrons must be at a sufficient energy level to move in the conduction zone. So, the space in a conductor is divided into two different types of regions: the network of conduction zone and isolated cells around individual molecules, somewhat like cement and pebbles in a piece of concrete. A conduction zone does not always appear in all materials. It is necessary for conductors, but absent in insulators. A superconductor is a special conductor with valence orbitals intersecting the conduction zone. Therefore, the valence electrons move naturally in the conduction zone without the need for energy to elevate them to the conduction zone. It is important to note that the term conduction band refers to a different concept defined in band theory.














