Bacillus is a genus of Gram-positive, rod-shaped bacteria, a member of the phylum Firmicutes, with 266 named species. The term is also used to describe the shape (rod) of certain bacteria; and the plural Bacilli is the name of the class of bacteria to which this genus belongs. Bacillus species can be either obligate aerobes: oxygen dependent; or facultative anaerobes: having the ability to continue living in the absence of oxygen. Cultured Bacillus species test positive for the enzyme catalase if oxygen has been used or is present.

An endospore is a dormant, tough, and non-reproductive structure produced by some bacteria in the phylum Firmicutes. The name "endospore" is suggestive of a spore or seed-like form, but it is not a true spore. It is a stripped-down, dormant form to which the bacterium can reduce itself. Endospore formation is usually triggered by a lack of nutrients, and usually occurs in gram-positive bacteria. In endospore formation, the bacterium divides within its cell wall, and one side then engulfs the other. Endospores enable bacteria to lie dormant for extended periods, even centuries. There are many reports of spores remaining viable over 10,000 years, and revival of spores millions of years old has been claimed. There is one report of viable spores of Bacillus marismortui in salt crystals approximately 250 million years old. When the environment becomes more favorable, the endospore can reactivate itself from the vegetative state. Most types of bacteria cannot change to the endospore form. Examples of bacterial species that can form endospores include Bacillus cereus, Bacillus anthracis, Bacillus thuringiensis, Clostridium botulinum, and Clostridium tetani.

A unicellular organism, also known as a single-celled organism, is an organism that consists of a single cell, unlike a multicellular organism that consists of multiple cells. Unicellular organisms fall into two general categories: prokaryotic organisms and eukaryotic organisms. All prokaryotes are unicellular and are classified into bacteria and archaea. Many eukaryotes are multicellular, but many are unicellular such as protozoa, unicellular algae, and unicellular fungi. Unicellular organisms are thought to be the oldest form of life, with early protocells possibly emerging 3.8–4.0 billion years ago.

A microbiological culture, or microbial culture, is a method of multiplying microbial organisms by letting them reproduce in predetermined culture medium under controlled laboratory conditions. Microbial cultures are foundational and basic diagnostic methods used as a research tool in molecular biology.

Polymyxins are antibiotics. Polymyxins B and E are used in the treatment of Gram-negative bacterial infections. They work mostly by breaking up the bacterial cell membrane. They are part of a broader class of molecules called nonribosomal peptides.
Collaborative intelligence characterizes multi-agent, distributed systems where each agent, human or machine, is autonomously contributing to a problem solving network. Collaborative autonomy of organisms in their ecosystems makes evolution possible. Natural ecosystems, where each organism's unique signature is derived from its genetics, circumstances, behavior and position in its ecosystem, offer principles for design of next generation social networks to support collaborative intelligence, crowdsourcing individual expertise, preferences, and unique contributions in a problem solving process.

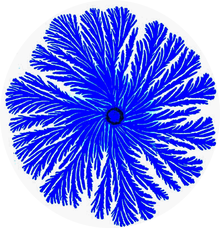
Paenibacillus is a genus of facultative anaerobic, endospore-forming bacteria, originally included within the genus Bacillus and then reclassified as a separate genus in 1993. Bacteria belonging to this genus have been detected in a variety of environments, such as: soil, water, rhizosphere, vegetable matter, forage and insect larvae, as well as clinical samples. The name reflects: Latin paene means almost, so the paenibacilli are literally "almost bacilli". The genus includes P. larvae, which causes American foulbrood in honeybees, P. polymyxa, which is capable of fixing nitrogen, so is used in agriculture and horticulture, the Paenibacillus sp. JDR-2 which is a rich source of chemical agents for biotechnology applications, and pattern-forming strains such as P. vortex and P. dendritiformis discovered in the early 90s, which develop complex colonies with intricate architectures as shown in the pictures:
Microbial intelligence is the intelligence shown by microorganisms. The concept encompasses complex adaptive behavior shown by single cells, and altruistic or cooperative behavior in populations of like or unlike cells mediated by chemical signalling that induces physiological or behavioral changes in cells and influences colony structures.

Myxococcus xanthus is a gram-negative, rod-shaped species of myxobacteria that exhibits various forms of self-organizing behavior in response to environmental cues. Under normal conditions with abundant food, it exists as a predatory, saprophytic single-species biofilm called a swarm. Under starvation conditions, it undergoes a multicellular development cycle.

Bacillus mycoides is a bacterium of the genus Bacillus. Like other Bacillus species, B. mycoides is Gram positive, rod-shaped, and forms spores. B. mycoides is distinguished from other Bacillus species by its unusual growth on agar plates, where it forms expansive hairy colonies with characteristic swirls.
Paenibacillus polymyxa, also known as Bacillus polymyxa, is a Gram-positive bacterium capable of fixing nitrogen. It is found in soil, plant tissues, marine sediments and hot springs. It may have a role in forest ecosystems and potential future applications as a biofertilizer and biocontrol agent in agriculture.

Bacteria are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among the first life forms to appear on Earth, and are present in most of its habitats. Bacteria inhabit soil, water, acidic hot springs, radioactive waste, and the deep biosphere of Earth's crust. Bacteria are vital in many stages of the nutrient cycle by recycling nutrients such as the fixation of nitrogen from the atmosphere. The nutrient cycle includes the decomposition of dead bodies; bacteria are responsible for the putrefaction stage in this process. In the biological communities surrounding hydrothermal vents and cold seeps, extremophile bacteria provide the nutrients needed to sustain life by converting dissolved compounds, such as hydrogen sulphide and methane, to energy. Bacteria also live in symbiotic and parasitic relationships with plants and animals. Most bacteria have not been characterised and there are many species that cannot be grown in the laboratory. The study of bacteria is known as bacteriology, a branch of microbiology.
Dictyostelium discoideum is a species of soil-dwelling amoeba belonging to the phylum Amoebozoa, infraphylum Mycetozoa. Commonly referred to as slime mold, D. discoideum is a eukaryote that transitions from a collection of unicellular amoebae into a multicellular slug and then into a fruiting body within its lifetime. Its unique asexual lifecycle consists of four stages: vegetative, aggregation, migration, and culmination. The lifecycle of D. discoideum is relatively short, which allows for timely viewing of all stages. The cells involved in the lifecycle undergo movement, chemical signaling, and development, which are applicable to human cancer research. The simplicity of its lifecycle makes D. discoideum a valuable model organism to study genetic, cellular, and biochemical processes in other organisms.

A prokaryote is a unicellular organism that lacks a nuclear membrane-enclosed nucleus. The word prokaryote comes from the Greek πρό and κάρυον. In the two-empire system arising from the work of Édouard Chatton, prokaryotes were classified within the empire Prokaryota. But in the three-domain system, based upon molecular analysis, prokaryotes are divided into two domains: Bacteria and Archaea. Organisms with nuclei are placed in a third domain, Eukaryota. In the study of the origins of life, prokaryotes are thought to have arisen before eukaryotes.

In biology, an organism is any organic, living system that functions as an individual entity. All organisms are composed of cells. Organisms are classified by taxonomy into groups such as multicellular animals, plants, and fungi; or unicellular microorganisms such as protists, bacteria, and archaea. All types of organisms are capable of reproduction, growth and development, maintenance, and some degree of response to stimuli. Beetles, squids, tetrapods, mushrooms, and vascular plants are examples of multicellular organisms that differentiate specialized tissues and organs during development.

Paenibacillus vortex is a species of pattern-forming bacteria, first discovered in the early 1990s by Eshel Ben-Jacob's group at Tel Aviv University. It is a social microorganism that forms colonies with complex and dynamic architectures. P. vortex is mainly found in heterogeneous and complex environments, such as the rhizosphere, the soil region directly influenced by plant roots.
Microorganisms engage in a wide variety of social interactions, including cooperation. A cooperative behavior is one that benefits an individual other than the one performing the behavior. This article outlines the various forms of cooperative interactions seen in microbial systems, as well as the benefits that might have driven the evolution of these complex behaviors.

Eshel Ben-Jacob, was a theoretical and experimental physicist at Tel Aviv University, holder of the Maguy-Glass Chair in Physics of Complex Systems, and Fellow of the Center for Theoretical Biological Physics (CTBP) at Rice University. During the 1980s he became a leader in the theory of self-organization and pattern formation in open systems, later extending this work to adaptive complex systems and biocomplexity. In the late 1980s, he turned to study of bacterial self-organization, He developed new pattern forming bacteria species, becoming a pioneer in the study of bacterial intelligence and social behaviors of bacteria.
Paenibacillus alvei is a species of bacteria within the order Bacillales. Like other species within the genus Paenibacillus, strains of this species grow in novel, vortex-like, or branched patterns. This species is associated with the honey bee disease European foulbrood.
Dokdonia donghaensis is a strictly aerobic, gram-negative, phototrophic bacterium that thrives in marine environments. The organism can grow at a broad range of temperatures on seawater media. It has the ability to form biofilms, which increases the organism's resistance to antimicrobial agents, such as tetracycline.