Salen refers to a tetradentate C2-symmetric ligand synthesized from salicylaldehyde (sal) and ethylenediamine (en). It may also refer to a class of compounds, which are structurally related to the classical salen ligand, primarily bis-Schiff bases. Salen ligands are notable for coordinating a wide range of different metals, which they can often stabilise in various oxidation states. For this reason salen-type compounds are used as metal deactivators. Metal salen complexes also find use as catalysts.
A carbometallation is any reaction where a carbon-metal bond reacts with a carbon-carbon π-bond to produce a new carbon-carbon σ-bond and a carbon-metal σ-bond. The resulting carbon-metal bond can undergo further carbometallation reactions or it can be reacted with a variety of electrophiles including halogenating reagents, carbonyls, oxygen, and inorganic salts to produce different organometallic reagents. Carbometallations can be performed on alkynes and alkenes to form products with high geometric purity or enantioselectivity, respectively. Some metals prefer to give the anti-addition product with high selectivity and some yield the syn-addition product. The outcome of syn and anti- addition products is determined by the mechanism of the carbometallation.

Organozirconium chemistry is the science of exploring the properties, structure, and reactivity of organozirconium compounds, which are organometallic compounds containing chemical bonds between carbon and zirconium. Organozirconium compounds have been widely studied, in part because they are useful catalysts in Ziegler-Natta polymerization.

2,2′-Bis(2-indenyl) biphenyl is an organic compound with the formula [C6H4C9H7]2. The compound is the precursor, upon deprotonation, to ansa-metallocene complexes within the area of transition metal indenyl complexes.
Organogold chemistry is the study of compounds containing gold–carbon bonds. They are studied in academic research, but have not received widespread use otherwise. The dominant oxidation states for organogold compounds are I with coordination number 2 and a linear molecular geometry and III with CN = 4 and a square planar molecular geometry.

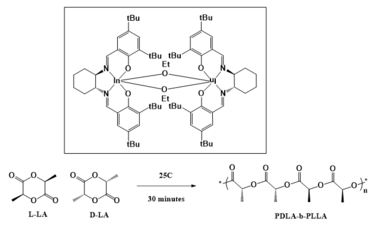
A metal salen complex is a coordination compound between a metal cation and a ligand derived from N,N′-bis(salicylidene)ethylenediamine, commonly called salen. The classical example is salcomine, the complex with divalent cobalt Co2+, usually denoted as Co(salen). These complexes are widely investigated as catalysts and enzyme mimics.
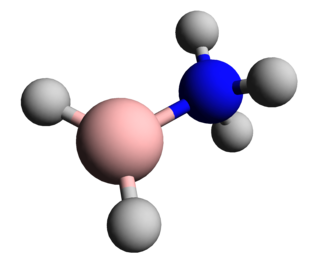
In chemistry, a boranylium ion is an inorganic cation with the chemical formula BR+
2, where R represents a non-specific substituent. Being electron-deficient, boranylium ions form adducts with Lewis bases. Boranylium ions have historical names that depend on the number of coordinated ligands:

Trisoxazolines are a class of tridentate, chiral ligands composed of three oxazoline rings. Despite being neutral they are able to form stable complexes with high oxidation state metals, such as rare earths, due to the chelate effect. The ligands have been investigated for molecular recognition and their complexes are used in asymmetric catalysts and polymerisation.
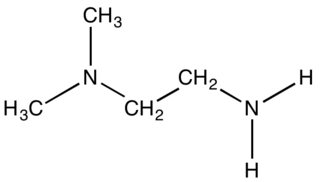
1,1-Dimethylethylenediamine is the organic compound with the formula (CH3)2N)CH2CH2NH2. It is a colorless liquid with a fishy odor. It features one primary amine and a tertiary amine. It is used to prepare a chelating diamine-containing ligands for the preparation of metal catalysts. It is a precursor to the drug chloropyramine.
In chemistry, compounds of palladium(III) feature the noble metal palladium in the unusual +3 oxidation state (in most of its compounds, palladium has the oxidation state II). Compounds of Pd(III) occur in mononuclear and dinuclear forms. Palladium(III) is most often invoked, not observed in mechanistic organometallic chemistry.
Clark Landis is an American chemist, whose research focuses on organic and inorganic chemistry. He is currently a Professor of Chemistry at the University of Wisconsin–Madison. He was awarded the ACS Award in Organometallic Chemistry in 2010, and is a fellow of the American Chemical Society and the American Association for the Advancement of Science.
William B. Tolman an American inorganic chemist focusing on the synthesis and characterization of model bioinorganic systems, and organometallic approaches towards polymer chemistry. He has served as Editor in Chief of the ACS journal Inorganic Chemistry, and as a Senior Investigator at the NSF Center for Sustainable Polymers. Tolman is a Fellow of the American Association for the Advancement of Science and the American Chemical Society.
A transition metal phosphido complex is a coordination complex containing a phosphido ligand (R2P, where R = H, organic substituent). With two lone pairs on phosphorus, the phosphido anion (R2P−) is comparable to an amido anion (R2N−), except that the M-P distances are longer and the phosphorus atom is more sterically accessible. For these reasons, phosphido is often a bridging ligand. The -PH2 ion or ligand is also called phosphanide or phosphido ligand.
β-Carbon elimination is a type of reaction in organometallic chemistry wherein an allyl ligand bonded to a metal center is broken into the corresponding metal-bonded alkyl (aryl) ligand and an alkene. It is a subgroup of elimination reactions. Though less common and less understood than β-hydride elimination, it is an important step involved in some olefin polymerization processes and transition-metal-catalyzed organic reactions.
2-Methylthioethylamine is the organosulfur compound with the formula CH3SCH2CH2NH2. It is a colorless liquid. It can be viewed as the product of S-methylation of cysteamine or decarboxylation of S-methylcysteine. The compound is a ligand and, via Schiff base condensations, a ligand precursor.
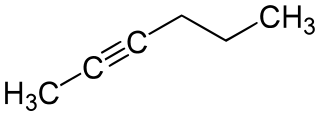
2-Hexyne is an organic compound that belongs to the alkyne group. Just like its isomers, it also has the chemical formula of C6H10.

Paula L. Diaconescu is a Romanian-American chemistry professor at the University of California, Los Angeles. She is known for her research on the synthesis of redox active transition metal complexes, the synthesis of lanthanide complexes, metal-induced small molecule activation, and polymerization reactions. She is a fellow of the American Association for the Advancement of Science.

Transition metal porphyrin complexes are a family of coordination complexes of the conjugate base of porphyrins. Iron porphyrin complexes occur widely in Nature, which has stimulated extensive studies on related synthetic complexes. The metal-porphyrin interaction is a strong one such that metalloporphyrins are thermally robust. They are catalysts and exhibit rich optical properties, although these complexes remain mainly of academic interest.
In chemistry, a redox switch is a molecular device, which has two subunits, a functional component and a control component. The "control subunit" is redox-active, meaning that it can exist in either of two redox states. The "functional" component could have a variety of readouts, such as fluorescence, the binding of a substrate, or catalytic activity. The key feature of such redox switches is that the functional component is influenced by the control subunit. One of many examples of a redox switch consists of an anthracene substituent to a copper-thiacrown ether (14-ane-4) coordination complex. When in the cupric oxidation state, the anthracene does not fluoresce. When in the cuprous state, the assembly is highly fluorescent. Several redox switches have been produced from ferrocenecarboxylic acid, which can be conjugated to a number of functional components. 1,1'-Diaminoferrocene has been incorporated into various diamide and diimine ligands, which form catalysts that exhibit redox switching.
1,1'-Diaminoferrocene is the organoiron compound with the formula Fe(C5H4NH2)2. It is the simplest diamine derivative of ferrocene. It is a yellow, air-sensitive solid that is soluble in aqueous acid. The 1,1' part of its name refers to the location of the amine groups on separate rings. Compared to the parent ferrocene, the diamine is about 600 mV more reducing.