
Dolly was a female Finnish Dorset sheep and the first mammal cloned from an adult somatic cell. She was cloned by associates of the Roslin Institute in Scotland, using the process of nuclear transfer from a cell taken from a mammary gland. Her cloning proved that a cloned organism could be produced from a mature cell from a specific body part. Contrary to popular belief, she was not the first animal to be cloned.
Human cloning is the creation of a genetically identical copy of a human. The term is generally used to refer to artificial human cloning, which is the reproduction of human cells and tissue. It does not refer to the natural conception and delivery of identical twins. The possibility of human cloning has raised controversies. These ethical concerns have prompted several nations to pass laws regarding human cloning.
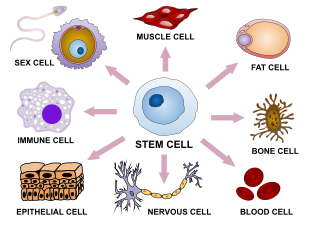
In multicellular organisms, stem cells are undifferentiated or partially differentiated cells that can differentiate into various types of cells and proliferate indefinitely to produce more of the same stem cell. They are the earliest type of cell in a cell lineage. They are found in both embryonic and adult organisms, but they have slightly different properties in each. They are usually distinguished from progenitor cells, which cannot divide indefinitely, and precursor or blast cells, which are usually committed to differentiating into one cell type.
Transdifferentiation, also known as lineage reprogramming, is an artificial process in which one mature somatic cell is transformed into another mature somatic cell without undergoing an intermediate pluripotent state or progenitor cell type. It is a type of metaplasia, which includes all cell fate switches, including the interconversion of stem cells. Current uses of transdifferentiation include disease modeling and drug discovery and in the future may include gene therapy and regenerative medicine. The term 'transdifferentiation' was originally coined by Selman and Kafatos in 1974 to describe a change in cell properties as cuticle producing cells became salt-secreting cells in silk moths undergoing metamorphosis.
Cellular differentiation is the process in which a stem cell alters from one type to a differentiated one Usually, the cell changes to a more specialized type. Differentiation happens multiple times during the development of a multicellular organism as it changes from a simple zygote to a complex system of tissues and cell types. Differentiation continues in adulthood as adult stem cells divide and create fully differentiated daughter cells during tissue repair and during normal cell turnover. Some differentiation occurs in response to antigen exposure. Differentiation dramatically changes a cell's size, shape, membrane potential, metabolic activity, and responsiveness to signals. These changes are largely due to highly controlled modifications in gene expression and are the study of epigenetics. With a few exceptions, cellular differentiation almost never involves a change in the DNA sequence itself. Although metabolic composition does get altered quite dramatically where stem cells are characterized by abundant metabolites with highly unsaturated structures whose levels decrease upon differentiation. Thus, different cells can have very different physical characteristics despite having the same genome.

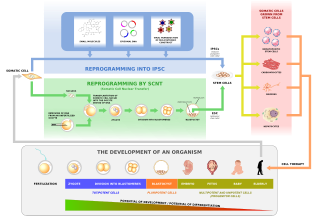
In genetics and developmental biology, somatic cell nuclear transfer (SCNT) is a laboratory strategy for creating a viable embryo from a body cell and an egg cell. The technique consists of taking an enucleated oocyte and implanting a donor nucleus from a somatic (body) cell. It is used in both therapeutic and reproductive cloning. In 1996, Dolly the sheep became famous for being the first successful case of the reproductive cloning of a mammal. In January 2018, a team of scientists in Shanghai announced the successful cloning of two female crab-eating macaques from foetal nuclei.
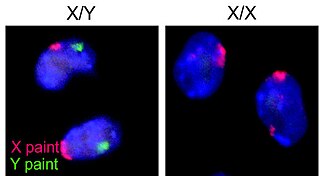
A germ cell is any biological cell that gives rise to the gametes of an organism that reproduces sexually. In many animals, the germ cells originate in the primitive streak and migrate via the gut of an embryo to the developing gonads. There, they undergo meiosis, followed by cellular differentiation into mature gametes, either eggs or sperm. Unlike animals, plants do not have germ cells designated in early development. Instead, germ cells can arise from somatic cells in the adult, such as the floral meristem of flowering plants.
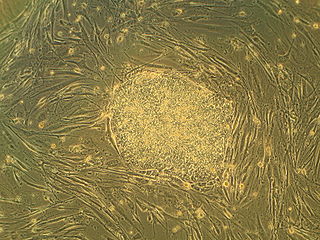
Embryonic stem cells are pluripotent stem cells derived from the inner cell mass of a blastocyst, an early-stage pre-implantation embryo. Human embryos reach the blastocyst stage 4–5 days post fertilization, at which time they consist of 50–150 cells. Isolating the embryoblast, or inner cell mass (ICM) results in destruction of the blastocyst, a process which raises ethical issues, including whether or not embryos at the pre-implantation stage have the same moral considerations as embryos in the post-implantation stage of development.

Sir John Bertrand Gurdon is a British developmental biologist. He is best known for his pioneering research in nuclear transplantation and cloning. He was awarded the Lasker Award in 2009. In 2012, he and Shinya Yamanaka were awarded the Nobel Prize for Physiology or Medicine for the discovery that mature cells can be converted to stem cells.

Oct-4, also known as POU5F1, is a protein that in humans is encoded by the POU5F1 gene. Oct-4 is a homeodomain transcription factor of the POU family. It is critically involved in the self-renewal of undifferentiated embryonic stem cells. As such, it is frequently used as a marker for undifferentiated cells. Oct-4 expression must be closely regulated; too much or too little will cause differentiation of the cells.

Nuclear transfer is a form of cloning. The steps involve removing the DNA from an oocyte, and injecting the nucleus which contains the DNA to be cloned. In rare instances, the newly constructed cell will divide normally, replicating the new DNA while remaining in a pluripotent state. If the cloned cells are placed in the uterus of a female mammal, a cloned organism develops to term in rare instances. This is how Dolly the Sheep and many other species were cloned. Cows are commonly cloned to select those that have the best milk production. On 24 January 2018, two monkey clones were reported to have been created with the technique for the first time.
In biology, reprogramming refers to erasure and remodeling of epigenetic marks, such as DNA methylation, during mammalian development or in cell culture. Such control is also often associated with alternative covalent modifications of histones.
The stem cell controversy is the consideration of the ethics of research involving the development and use of human embryos. Most commonly, this controversy focuses on embryonic stem cells. Not all stem cell research involves human embryos. For example, adult stem cells, amniotic stem cells, and induced pluripotent stem cells do not involve creating, using, or destroying human embryos, and thus are minimally, if at all, controversial. Many less controversial sources of acquiring stem cells include using cells from the umbilical cord, breast milk, and bone marrow, which are not pluripotent.

Induced pluripotent stem cells are a type of pluripotent stem cell that can be generated directly from a somatic cell. The iPSC technology was pioneered by Shinya Yamanaka’s lab in Kyoto, Japan, who showed in 2006 that the introduction of four specific genes, collectively known as Yamanaka factors, encoding transcription factors could convert somatic cells into pluripotent stem cells. He was awarded the 2012 Nobel Prize along with Sir John Gurdon "for the discovery that mature cells can be reprogrammed to become pluripotent."

Shinya Yamanaka is a Japanese stem cell researcher, winner of the Nobel Prize. He serves as the director of Center for iPS Cell Research and Application and a professor at the Institute for Frontier Medical Sciences at Kyoto University; as a senior investigator at the UCSF-affiliated Gladstone Institutes in San Francisco, California; and as a professor of anatomy at University of California, San Francisco (UCSF). Yamanaka is also a past president of the International Society for Stem Cell Research (ISSCR).
Embryomics is the identification, characterization and study of the diverse cell types which arise during embryogenesis, especially as this relates to the location and developmental history of cells in the embryo. Cell type may be determined according to several criteria: location in the developing embryo, gene expression as indicated by protein and nucleic acid markers and surface antigens, and also position on the embryogenic tree.

Cell potency is a cell's ability to differentiate into other cell types. The more cell types a cell can differentiate into, the greater its potency. Potency is also described as the gene activation potential within a cell, which like a continuum, begins with totipotency to designate a cell with the most differentiation potential, pluripotency, multipotency, oligopotency, and finally unipotency.
Embryonic stem cells are capable of self-renewing and differentiating to the desired fate depending on their position in the body. Stem cell homeostasis is maintained through epigenetic mechanisms that are highly dynamic in regulating the chromatin structure as well as specific gene transcription programs. Epigenetics has been used to refer to changes in gene expression, which are heritable through modifications not affecting the DNA sequence.
Induced stem cells (iSC) are stem cells derived from somatic, reproductive, pluripotent or other cell types by deliberate epigenetic reprogramming. They are classified as either totipotent (iTC), pluripotent (iPSC) or progenitor or unipotent – (iUSC) according to their developmental potential and degree of dedifferentiation. Progenitors are obtained by so-called direct reprogramming or directed differentiation and are also called induced somatic stem cells.

Glis1 is gene encoding a Krüppel-like protein of the same name whose locus is found on Chromosome 1p32.3. The gene is enriched in unfertilised eggs and embryos at the one cell stage and it can be used to promote direct reprogramming of somatic cells to induced pluripotent stem cells, also known as iPS cells. Glis1 is a highly promiscuous transcription factor, regulating the expression of numerous genes, either positively or negatively. In organisms, Glis1 does not appear to have any directly important functions. Mice whose Glis1 gene has been removed have no noticeable change to their phenotype.














