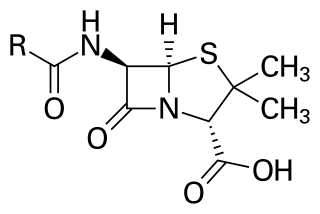
Penicillins are a group of antibiotics originally obtained from Penicillium moulds, principally P. chrysogenum and P. rubens. Most penicillins in clinical use are chemically synthesised from naturally-produced penicillins. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: penicillin G and penicillin V. Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococci and streptococci. They are members of the β-lactam antibiotics, which are some of the most powerful and successful achievements in modern science. They are still widely used today for different bacterial infections, though many types of bacteria have developed resistance following extensive use.
Peptidoglycan or murein is a polymer consisting of sugars and amino acids that forms a mesh-like layer outside the plasma membrane of most bacteria, forming the cell wall. The sugar component consists of alternating residues of β-(1,4) linked N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM). Attached to the N-acetylmuramic acid is a peptide chain of three to five amino acids. The peptide chain can be cross-linked to the peptide chain of another strand forming the 3D mesh-like layer. Peptidoglycan serves a structural role in the bacterial cell wall, giving structural strength, as well as counteracting the osmotic pressure of the cytoplasm. Peptidoglycan is also involved in binary fission during bacterial cell reproduction.
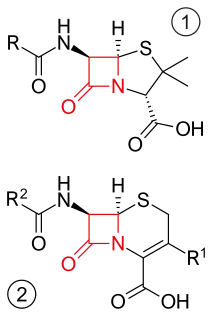
β-lactam antibiotics are antibiotics that contain a beta-lactam ring in their molecular structure. This includes penicillin derivatives (penams), cephalosporins (cephems), monobactams, carbapenems and carbacephems. Most β-lactam antibiotics work by inhibiting cell wall biosynthesis in the bacterial organism and are the most widely used group of antibiotics. Until 2003, when measured by sales, more than half of all commercially available antibiotics in use were β-lactam compounds. The first β-lactam antibiotic discovered, penicillin, was isolated from a rare variant of Penicillium notatum.

Cefalexin, also spelled cephalexin, is an antibiotic that can treat a number of bacterial infections. It kills gram-positive and some gram-negative bacteria by disrupting the growth of the bacterial cell wall. Cefalexin is a beta-lactam antibiotic within the class of first-generation cephalosporins. It works similarly to other agents within this class, including intravenous cefazolin, but can be taken by mouth.

Peter Dennis Mitchell, FRS was a British biochemist who was awarded the 1978 Nobel Prize for Chemistry for his discovery of the chemiosmotic mechanism of ATP synthesis.

Robert William Holley was an American biochemist. He shared the Nobel Prize in Physiology or Medicine in 1968 for describing the structure of alanine transfer RNA, linking DNA and protein synthesis.

Ticarcillin is a carboxypenicillin. It is almost always sold and used in combination with clavulanate as ticarcillin/clavulanic acid. Because it is a penicillin, it also falls within the larger class of beta-lactam antibiotics. Its main clinical use is as an injectable antibiotic for the treatment of Gram-negative bacteria, particularly Pseudomonas aeruginosa. It is also one of the few antibiotics capable of treating Stenotrophomonas maltophilia infections.

In biochemistry, suicide inhibition, also known as suicide inactivation or mechanism-based inhibition, is an irreversible form of enzyme inhibition that occurs when an enzyme binds a substrate analog and forms an irreversible complex with it through a covalent bond during the normal catalysis reaction. The inhibitor binds to the active site where it is modified by the enzyme to produce a reactive group that reacts irreversibly to form a stable inhibitor-enzyme complex. This usually uses a prosthetic group or a coenzyme, forming electrophilic alpha and beta unsaturated carbonyl compounds and imines.

Flucloxacillin, also known as floxacillin, is an antibiotic used to treat skin infections, external ear infections, infections of leg ulcers, diabetic foot infections, and infection of bone. It may be used together with other medications to treat pneumonia, and endocarditis. It may also be used prior to surgery to prevent Staphylococcus infections. It is not effective against methicillin-resistant Staphylococcus aureus (MRSA). It is taken by mouth or given by injection into a vein or muscle.
Penicillin-binding proteins (PBPs) are a group of proteins that are characterized by their affinity for and binding of penicillin. They are a normal constituent of many bacteria; the name just reflects the way by which the protein was discovered. All β-lactam antibiotics bind to PBPs, which are essential for bacterial cell wall synthesis. PBPs are members of a subgroup of enzymes called transpeptidases. Specifically, PBPs are DD-transpeptidases.

Ralph Alexander Raphael was a British organic chemist, well known for his use of acteylene derivatives in the synthesis of natural products with biological activity.

Oxacillin is a narrow-spectrum beta-lactam antibiotic of the penicillin class developed by Beecham.

Tropolone is an organic compound with the formula C7H5(OH)O. It is a pale yellow solid that is soluble in organic solvents. The compound has been of interest to research chemists because of its unusual electronic structure and its role as a ligand precursor. Although not usually prepared from tropone, it can be viewed as its derivative with a hydroxyl group in the 2-position.

6-APA is an abbreviation used for the name of the chemical compound (+)-6-aminopenicillanic acid. In 1958, Beecham scientists from Brockham Park, Surrey, found a way to obtain 6-APA from penicillin. Other β-lactam antibiotics could then be synthesized by attaching various side-chains to the nucleus. The major commercial source of 6-APA is still natural penicillin G: the semi-synthetic penicillins derived from 6-APA are also referred to as penicillins and are considered part of the penicillin family of antibiotics.

Aminohippuric acid or para-aminohippuric acid (PAH), a derivative of hippuric acid, is a diagnostic agent useful in medical tests involving the kidney used in the measurement of renal plasma flow. It is an amide derivative of the amino acid glycine and para-aminobenzoic acid that is not naturally found in humans; it needs to be IV infused before diagnostic use.

Talampicillin is a beta lactam antibiotic from the penicillin family. It is an acid stable prodrug that was administered orally. It is not approved by the FDA for use in the United States. It should be avoided in Liver diseases
John Clark Sheehan was an American organic chemist whose work on synthetic penicillin led to tailor-made forms of the drug. After nine years of hard work at the Massachusetts Institute of Technology (M.I.T.), he became the first to discover a practical method for synthesizing penicillin V. While achieving total synthesis, Sheehan also produced an intermediate compound, 6-aminopenicillanic acid, which turned out to be the foundation of hundreds of kinds of synthetic penicillin. Dr. Sheehan's research on synthetic penicillin paved the way for the development of customized forms of the lifesaving antibiotic that target specific bacteria. Over the four decades he worked at M.I.T., Sheehan came to hold over 30 patents, including the invention of ampicillin, a commonly used semi-synthetic penicillin that is taken orally rather than by injection. His research covered not only penicillin, but also peptides, other antibiotics, alkaloids, and steroids.
Gertrude Maud Robinson was an influential organic chemist most famous for her work on plant pigments; the Piloty-Robinson Pyrrole Synthesis, which is named for her; her syntheses of fatty acids; and her synthesis of δ-hexenolactone, the first synthetic molecule with the character of penicillin.
Penicillium fennelliae is an anamorph species of the genus of Penicillium which produces patulin, orsellinic acid and penicillinic acid.

Tetronic acid is a chemical compound, classified as a γ-lactone, with the molecular formula C4H4O3.

















