The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Berkelium is a transuranic radioactive chemical element with the symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium, plutonium, curium and americium.

Curium is a transuranic, radioactive chemical element with the symbol Cm and atomic number 96. This actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first intentionally made by the team of Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944, using the cyclotron at Berkeley. They bombarded the newly discovered element plutonium with alpha particles. This was then sent to the Metallurgical Laboratory at University of Chicago where a tiny sample of curium was eventually separated and identified. The discovery was kept secret until after the end of World War II. The news was released to the public in November 1947. Most curium is produced by bombarding uranium or plutonium with neutrons in nuclear reactors – one tonne of spent nuclear fuel contains ~20 grams of curium.
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity, as in the case of zinc and its use as a maintenance therapy to prevent the absorption of copper in people with Wilson's disease.

Ethylenediaminetetraacetic acid (EDTA), also called edetic acid after its own abbreviation, is an aminopolycarboxylic acid with the formula [CH2N(CH2CO2H)2]2. This white, water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-soluble complexes even at neutral pH. It is thus used to dissolve Fe- and Ca-containing scale as well as to deliver iron ions under conditions where its oxides are insoluble. EDTA is available as several salts, notably disodium EDTA, sodium calcium edetate, and tetrasodium EDTA, but these all function similarly.

An oxidizing agent is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent. In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide and the halogens.

Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, the purification of chemicals and separation of substances.

Gadolinium(III) chloride, also known as gadolinium trichloride, is GdCl3. It is a colorless, hygroscopic, water-soluble solid. The hexahydrate GdCl3∙6H2O is commonly encountered and is sometimes also called gadolinium trichloride. Gd3+ species are of special interest because the ion has the maximum number of unpaired spins possible, at least for known elements. With seven valence electrons and seven available f-orbitals, all seven electrons are unpaired and symmetrically arranged around the metal. The high magnetism and high symmetry combine to make Gd3+ a useful component in NMR spectroscopy and MRI.
A complexometric indicator is an ionochromic dye that undergoes a definite color change in presence of specific metal ions. It forms a weak complex with the ions present in the solution, which has a significantly different color from the form existing outside the complex. Complexometric indicators are also known as pM indicators.
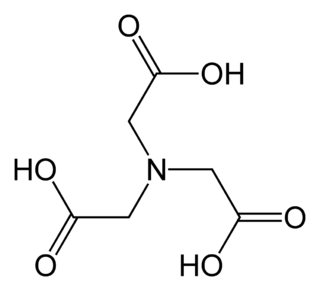
Nitrilotriacetic acid (NTA) is the aminopolycarboxylic acid with the formula N(CH2CO2H)3. It is a colourless solid that is used as a chelating agent, which forms coordination compounds with metal ions (chelates) such as Ca2+, Co2+, Cu2+, and Fe3+.

Bleach is the generic name for any chemical product that is used industrially or domestically to remove colour (whitening) from fabric or fiber or to clean or to remove stains in a process called bleaching. It often refers specifically to a dilute solution of sodium hypochlorite, also called "liquid bleach".
Bleaching of wood pulp is the chemical processing of wood pulp to lighten its color and whiten the pulp. The primary product of wood pulp is paper, for which whiteness is an important characteristic. These processes and chemistry are also applicable to the bleaching of non-wood pulps, such as those made from bamboo or kenaf.

EDDHA or ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid) is a chelating agent. Like EDTA, it binds metal ions as a hexadentate ligand, using two amines, two phenolate centers, and two carboxylates as the six binding sites. The complexes are typically anionic. The ligand itself is a white, water soluble powder. Both the free ligand and its tetraanionic chelating agent are abbreviated EDDHA. In contrast to EDDHA, most related aminopolycarboxylic acid chelating agents feature tertiary amines and few have phenolate groups.
In coordination chemistry, a stability constant is an equilibrium constant for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the complex. There are two main kinds of complex: compounds formed by the interaction of a metal ion with a ligand and supramolecular complexes, such as host–guest complexes and complexes of anions. The stability constant(s) provide(s) the information required to calculate the concentration(s) of the complex(es) in solution. There are many areas of application in chemistry, biology and medicine.

Isosaccharinic acid (ISA) is a six-carbon sugar acid which is formed by the action of calcium hydroxide on lactose and other carbohydrates. It is of interest because it may form in intermediate-level nuclear waste stores when cellulose is degraded by the calcium hydroxide in cements such as Portland cement. The calcium salt of the alpha form of ISA is very crystalline and quite insoluble in cold water, but in hot water it is soluble.

Many compounds of thorium are known: this is because thorium and uranium are the most stable and accessible actinides and are the only actinides that can be studied safely and legally in bulk in a normal laboratory. As such, they have the best-known chemistry of the actinides, along with that of plutonium, as the self-heating and radiation from them is not enough to cause radiolysis of chemical bonds as it is for the other actinides. While the later actinides from americium onwards are predominantly trivalent and behave more similarly to the corresponding lanthanides, as one would expect from periodic trends, the early actinides up to plutonium have relativistically destabilised and hence delocalised 5f and 6d electrons that participate in chemistry in a similar way to the early transition metals of group 3 through 8: thus, all their valence electrons can participate in chemical reactions, although this is not common for neptunium and plutonium.

Ferric EDTA is the coordination complex formed from ferric ions and EDTA. EDTA has a high affinity for ferric ions. It is a yellow solid that gives yellowish aqueous solutions.
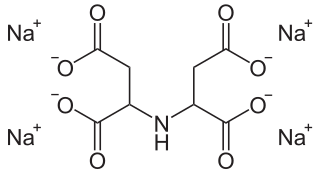
Tetrasodium iminodisuccinate is a sodium salt of iminodisuccinic acid, also referred to as N-(1,2-dicarboxyethyl)aspartic acid.
Chelated platinum is an ionized form of platinum that forms two or more bonds with a counter ion. Some platinum chelates are claimed to have antimicrobial activity.













