
A pilus is a hair-like appendage found on the surface of many bacteria and archaea. The terms pilus and fimbria can be used interchangeably, although some researchers reserve the term pilus for the appendage required for bacterial conjugation. All conjugative pili are primarily composed of pilin – fibrous proteins, which are oligomeric.

Mannans are polymers containing the sugar mannose as a principal component. They are a type of polysaccharide found in hemicellulose, a major source of biomass found in higher plants such as softwoods. These polymers also typically contain two other sugars, galactose and glucose. They are often branched.

Vibrio is a genus of Gram-negative bacteria, possessing a curved-rod (comma) shape, several species of which can cause foodborne infection, usually associated with eating undercooked seafood. Being highly salt tolerant and unable to survive in fresh water, Vibrio spp. are commonly found in various salt water environments. Vibrio spp. are facultative anaerobes that test positive for oxidase and do not form spores. All members of the genus are motile. They are able to have polar or lateral flagellum with or without sheaths. Vibrio species typically possess two chromosomes, which is unusual for bacteria. Each chromosome has a distinct and independent origin of replication, and are conserved together over time in the genus. Recent phylogenies have been constructed based on a suite of genes.

Sialic acids are a class of alpha-keto acid sugars with a nine-carbon backbone. The term "sialic acid" was first introduced by Swedish biochemist Gunnar Blix in 1952. The most common member of this group is N-acetylneuraminic acid found in animals and some prokaryotes.

Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent activities, corresponding to ~4% of all classified activities. The versatility of PLP arises from its ability to covalently bind the substrate, and then to act as an electrophilic catalyst, thereby stabilizing different types of carbanionic reaction intermediates.

Teicoplanin is an antibiotic used in the prophylaxis and treatment of serious infections caused by Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus and Enterococcus faecalis. It is a semisynthetic glycopeptide antibiotic with a spectrum of activity similar to vancomycin. Its mechanism of action is to inhibit bacterial cell wall synthesis.

Clavulanic acid is a β-lactam drug that functions as a mechanism-based β-lactamase inhibitor. While not effective by itself as an antibiotic, when combined with penicillin-group antibiotics, it can overcome antibiotic resistance in bacteria that secrete β-lactamase, which otherwise inactivates most penicillins.

Lincomycin is a lincosamide antibiotic that comes from the actinomycete Streptomyces lincolnensis. A related compound, clindamycin, is derived from lincomycin by using thionyl chloride to replace the 7-hydroxy group with a chlorine atom with inversion of chirality. It was released for medical use in September 1964.
Mycolic acids are long fatty acids found in the cell walls of the Mycolata taxon, a group of bacteria that includes Mycobacterium tuberculosis, the causative agent of the disease tuberculosis. They form the major component of the cell wall of mycolata species. Despite their name, mycolic acids have no biological link to fungi; the name arises from the filamentous appearance their presence gives mycolata under high magnification. The presence of mycolic acids in the cell wall also gives mycolata a distinct gross morphological trait known as "cording". Mycolic acids were first isolated by Stodola et al. in 1938 from an extract of M. tuberculosis.

Glycosyltransferases are enzymes that establish natural glycosidic linkages. They catalyze the transfer of saccharide moieties from an activated nucleotide sugar to a nucleophilic glycosyl acceptor molecule, the nucleophile of which can be oxygen- carbon-, nitrogen-, or sulfur-based.

Colitose is a mannose-derived 3,6-dideoxysugar produced by certain bacteria. It is a constituent of the lipopolysaccharide. It is the enantiomer of abequose.
In biochemistry, fatty acid synthesis is the creation of fatty acids from acetyl-CoA and NADPH through the action of enzymes called fatty acid synthases. This process takes place in the cytoplasm of the cell. Most of the acetyl-CoA which is converted into fatty acids is derived from carbohydrates via the glycolytic pathway. The glycolytic pathway also provides the glycerol with which three fatty acids can combine to form triglycerides, the final product of the lipogenic process. When only two fatty acids combine with glycerol and the third alcohol group is phosphorylated with a group such as phosphatidylcholine, a phospholipid is formed. Phospholipids form the bulk of the lipid bilayers that make up cell membranes and surrounds the organelles within the cells.

The enzyme GDP-mannose 4,6-dehydratase (EC 4.2.1.47) catalyzes the chemical reaction
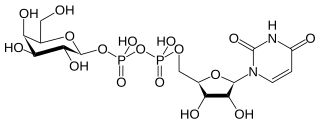
In nucleotide sugar metabolism a group of biochemicals known as nucleotide sugars act as donors for sugar residues in the glycosylation reactions that produce polysaccharides. They are substrates for glycosyltransferases. The nucleotide sugars are also intermediates in nucleotide sugar interconversions that produce some of the activated sugars needed for glycosylation reactions. Since most glycosylation takes place in the endoplasmic reticulum and golgi apparatus, there are a large family of nucleotide sugar transporters that allow nucleotide sugars to move from the cytoplasm, where they are produced, into the organelles where they are consumed.

Guanosine diphosphate mannose or GDP-mannose is a nucleotide sugar that is a substrate for glycosyltransferase reactions in metabolism. This compound is a substrate for enzymes called mannosyltransferases.
Vibriocins are a group of bacteriocins produced by, and active against, gram-negative bacteria in the genus Vibrio. They were first discovered in 1962, considerably after the original bacteriocins, the colicins, which were discovered in 1925.
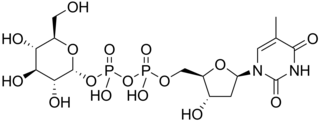
Thymidine diphosphate glucose is a nucleotide-linked sugar consisting of deoxythymidine diphosphate linked to glucose. It is the starting compound for the syntheses of many deoxysugars.
Core oligosaccharide is a short chain of sugar residues within Gram-negative lipopolysaccharide (LPS). Core-OS are highly diverse among bacterial species and even within strains of species
Interspecies quorum sensing is a type of quorum sensing in which bacteria send and receive signals to other species besides their own. This is accomplished by the secretion of signaling molecules which trigger a response in nearby bacteria at high enough concentrations. Once the molecule hits a certain concentration it triggers the transcription of certain genes such as virulence factors. It has been discovered that bacteria can not only interact via quorum sensing with members of their own species but that there is a kind of universal molecule that allows them to gather information about other species as well. This universal molecule is called autoinducer 2 or AI-2.
The alpha-D-phosphohexomutases are a large superfamily of enzymes, with members in all three domains of life. Enzymes from this superfamily are ubiquitous in organisms from E. Coli to humans, and catalyze a phosphoryl transfer reaction on a phosphosugar substrate. Four well studied subgroups in the superfamily are:
- Phosphoglucomutase (PGM)
- Phosphoglucomutase/Phosphomannomutase (PGM/PMM)
- Phosphoglucosamine mutase (PNGM)
- Phosphoaceytlglucosamine mutase (PAGM)














