
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining.
In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in their reference state, with all substances in their standard states. The standard pressure value p⦵ = 105 Pa(= 100 kPa = 1 bar) is recommended by IUPAC, although prior to 1982 the value 1.00 atm (101.325 kPa) was used. There is no standard temperature. Its symbol is ΔfH⦵. The superscript Plimsoll on this symbol indicates that the process has occurred under standard conditions at the specified temperature (usually 25 °C or 298.15 K).
Silane (Silicane) is an inorganic compound with chemical formula SiH4. It is a colourless, pyrophoric, toxic gas with a sharp, repulsive, pungent smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Silane with alkyl groups are effective water repellents for mineral surfaces such as concrete and masonry. Silanes with both organic and inorganic attachments are used as coupling agents. Silanes are commonly used to apply coatings to surfaces or as an adhesion promoter.
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities are on the right-hand side with a plus sign between the entities in both the reactants and the products, and an arrow that points towards the products to show the direction of the reaction. The chemical formulas may be symbolic, structural, or intermixed. The coefficients next to the symbols and formulas of entities are the absolute values of the stoichiometric numbers. The first chemical equation was diagrammed by Jean Beguin in 1615.
Methanogens are microorganisms that produce methane as a metabolic byproduct in hypoxic conditions. They are prokaryotic and belong to the domain Archaea. All known methanogens are members of the archaeal phylum Euryarchaeota. Methanogens are common in wetlands, where they are responsible for marsh gas, and can occur in the digestive tracts of animals including ruminants and humans, where they are responsible for the methane content of belching and flatulence. In marine sediments, the biological production of methane, termed methanogenesis, is generally confined to where sulfates are depleted below the top layers. Methanogenic archaea populations play an indispensable role in anaerobic wastewater treatments. Other methanogens are extremophiles, found in environments such as hot springs and submarine hydrothermal vents as well as in the "solid" rock of Earth's crust, kilometers below the surface.
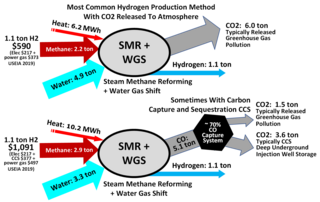
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is hydrogen production. The reaction is represented by this equilibrium:

The Sabatier reaction or Sabatier process produces methane and water from a reaction of hydrogen with carbon dioxide at elevated temperatures and pressures in the presence of a nickel catalyst. It was discovered by the French chemists Paul Sabatier and Jean-Baptiste Senderens in 1897. Optionally, ruthenium on alumina makes a more efficient catalyst. It is described by the following exothermic reaction:
In atmospheric chemistry, NOx is shorthand for nitric oxide and nitrogen dioxide, the nitrogen oxides that are most relevant for air pollution. These gases contribute to the formation of smog and acid rain, as well as affecting tropospheric ozone.
Methanation is the conversion of carbon monoxide and carbon dioxide (COx) to methane (CH4) through hydrogenation. The methanation reactions of COx were first discovered by Sabatier and Senderens in 1902.

Methane is a chemical compound with the chemical formula CH4. It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it poses technical challenges due to its gaseous state under normal conditions for temperature and pressure.
In combustion engineering and explosion studies, the Markstein number characterizes the effect of local heat release of a propagating flame on variations in the surface topology along the flame and the associated local flame front curvature. The dimensionless Markstein number is defined as:

Ishwar Kanwar Puri is an Indian-American and Canadian scientist, engineer, and academic.
Organoplatinum chemistry is the chemistry of organometallic compounds containing a carbon to platinum chemical bond, and the study of platinum as a catalyst in organic reactions. Organoplatinum compounds exist in oxidation state 0 to IV, with oxidation state II most abundant. The general order in bond strength is Pt-C (sp) > Pt-O > Pt-N > Pt-C (sp3). Organoplatinum and organopalladium chemistry are similar, but organoplatinum compounds are more stable and therefore less useful as catalysts.
Sulfur mononitride is an inorganic compound with the molecular formula SN. It is the sulfur analogue of and isoelectronic to the radical nitric oxide, NO. It was initially detected in 1975, in outer space in giant molecular clouds and later the coma of comets. This spurred further laboratory studies of the compound. Synthetically, it is produced by electric discharge in mixtures of nitrogen and sulfur compounds, or combustion in the gas phase and by photolysis in solution.
Micro-combustion is the sequence of exothermic chemical reaction between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species at micro level. The release of heat can result in the production of light in the form of either glowing or a flame. Fuels of interest often include organic compounds in the gas, liquid or solid phase. The major problem of micro-combustion is the high surface to volume ratio. As the surface to volume ratio increases heat loss to walls of combustor increases which leads to flame quenching.
The extended discrete element method (XDEM) is a numerical technique that extends the dynamics of granular material or particles as described through the classical discrete element method (DEM) by additional properties such as the thermodynamic state, stress/strain or electro-magnetic field for each particle. Contrary to a continuum mechanics concept, the XDEM aims at resolving the particulate phase with its various processes attached to the particles. While the discrete element method predicts position and orientation in space and time for each particle, the extended discrete element method additionally estimates properties such as internal temperature and/or species distribution or mechanical impact with structures.
In chemical kinetics, an intrinsic low-dimensional manifold is a technique to simplify the study of reaction mechanisms using dynamical systems, first proposed in 1992.

Techniques have been developed to produce carbon nanotubes in sizable quantities, including arc discharge, laser ablation, high-pressure carbon monoxide disproportionation, and chemical vapor deposition (CVD). Most of these processes take place in a vacuum or with process gases. CVD growth of CNTs can occur in vacuum or at atmospheric pressure. Large quantities of nanotubes can be synthesized by these methods; advances in catalysis and continuous growth are making CNTs more commercially viable.
Chemical looping reforming (CLR) and gasification (CLG) are the operations that involve the use of gaseous carbonaceous feedstock and solid carbonaceous feedstock, respectively, in their conversion to syngas in the chemical looping scheme. The typical gaseous carbonaceous feedstocks used are natural gas and reducing tail gas, while the typical solid carbonaceous feedstocks used are coal and biomass. The feedstocks are partially oxidized to generate syngas using metal oxide oxygen carriers as the oxidant. The reduced metal oxide is then oxidized in the regeneration step using air. The syngas is an important intermediate for generation of such diverse products as electricity, chemicals, hydrogen, and liquid fuels.
In the study of diffusion flame, Liñán's equation is a second-order nonlinear ordinary differential equation which describes the inner structure of the diffusion flame, first derived by Amable Liñán in 1974. The equation reads as








