Related Research Articles
Nanomedicine is the medical application of nanotechnology. Nanomedicine ranges from the medical applications of nanomaterials and biological devices, to nanoelectronic biosensors, and even possible future applications of molecular nanotechnology such as biological machines. Current problems for nanomedicine involve understanding the issues related to toxicity and environmental impact of nanoscale materials.

Nanomaterials describe, in principle, materials of which a single unit is sized between 1 and 100 nm.

A nanoshell, or rather a nanoshell plasmon, is a type of spherical nanoparticle consisting of a dielectric core which is covered by a thin metallic shell. These nanoshells involve a quasiparticle called a plasmon which is a collective excitation or quantum plasma oscillation where the electrons simultaneously oscillate with respect to all the ions.
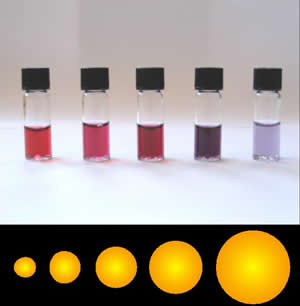
Colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. The colloid is coloured usually either wine red or blue-purple . Due to their optical, electronic, and molecular-recognition properties, gold nanoparticles are the subject of substantial research, with many potential or promised applications in a wide variety of areas, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine.

Photosensitizers are light absorbers that alter the course of a photochemical reaction. They usually are catalysts. They can function by many mechanisms, sometimes they donate an electron to the substrate, sometimes they abstract a hydrogen atom from the substrate. At the end of this process, the photosensitizer returns to its ground state, where it remains chemically intact, poised to absorb more light. One branch of chemistry which frequently utilizes photosensitizers is polymer chemistry, using photosensitizers in reactions such as photopolymerization, photocrosslinking, and photodegradation. Photosensitizers are also used to generate prolonged excited electronic states in organic molecules with uses in photocatalysis, photon upconversion and photodynamic therapy. Generally, photosensitizers absorb electromagnetic radiation consisting of infrared radiation, visible light radiation, and ultraviolet radiation and transfer absorbed energy into neighboring molecules. This absorption of light is made possible by photosensitizers' large de-localized π-systems, which lowers the energy of HOMO and LUMO orbitals to promote photoexcitation. While many photosensitizers are organic or organometallic compounds, there are also examples of using semiconductor quantum dots as photosensitizers.
Gold Nanocages are hollow, porous gold nanoparticles ranging in size from 10 to over 150 nm. They are created by reacting silver nanoparticles with chloroauric acid (HAuCl4) in boiling water. Whereas gold nanoparticles absorb light in the visible spectrum of light (at about 550 nm), gold nanocages absorb light in the near-infrared, where biological tissues absorb the least light. Because they are also biocompatible, gold nanocages are promising as a contrast agent for optical coherence tomography. Gold nanocages also absorb light and heat up (Photothermal effect), killing surrounding cancer cells. Nanocages have been functionalized with cancer-specific antibodies.

An artificial enzyme is a synthetic organic molecule or ion that recreates one or more functions of an enzyme. It seeks to deliver catalysis at rates and selectivity observed in naturally occurring enzymes.
Carbon nanotubes (CNTs) are very prevalent in today's world of medical research and are being highly researched in the fields of efficient drug delivery and biosensing methods for disease treatment and health monitoring. Carbon nanotube technology has shown to have the potential to alter drug delivery and biosensing methods for the better, and thus, carbon nanotubes have recently garnered interest in the field of medicine.
Multiple layered plasmonics use electronically responsive media to change and manipulate the plasmonic properties of plasmons. The properties typically being manipulated can include the directed scattering of light and light absorption. The use of these to use “changeable” plasmonics is currently undergoing development in the academic community by allowing them to have multiple sets of functions that are dependent on how they are being manipulated or excited. Under these new manipulations, such as multiple layers that respond to different resonant frequencies, their new functions were designed to accomplish multiple objectives in a single application.

Plasmonic nanoparticles are particles whose electron density can couple with electromagnetic radiation of wavelengths that are far larger than the particle due to the nature of the dielectric-metal interface between the medium and the particles: unlike in a pure metal where there is a maximum limit on what size wavelength can be effectively coupled based on the material size.
Photoimmunotherapy (PIT) is an oncological treatment that combines photodynamic therapy of tumor with immunotherapy treatment. Combining photodynamic therapy with immunotherapy enhances the immunostimulating response and has synergistic effects for metastatic cancer treatment.
The applications of nanotechnology, commonly incorporate industrial, medicinal, and energy uses. These include more durable construction materials, therapeutic drug delivery, and higher density hydrogen fuel cells that are environmentally friendly. Being that nanoparticles and nanodevices are highly versatile through modification of their physiochemical properties, they have found uses in nanoscale electronics, cancer treatments, vaccines, hydrogen fuel cells, and nanographene batteries.

A localized surface plasmon (LSP) is the result of the confinement of a surface plasmon in a nanoparticle of size comparable to or smaller than the wavelength of light used to excite the plasmon. When a small spherical metallic nanoparticle is irradiated by light, the oscillating electric field causes the conduction electrons to oscillate coherently. When the electron cloud is displaced relative to its original position, a restoring force arises from Coulombic attraction between electrons and nuclei. This force causes the electron cloud to oscillate. The oscillation frequency is determined by the density of electrons, the effective electron mass, and the size and shape of the charge distribution. The LSP has two important effects: electric fields near the particle's surface are greatly enhanced and the particle's optical absorption has a maximum at the plasmon resonant frequency. Surface plasmon resonance can also be tuned based on the shape of the nanoparticle. The plasmon frequency can be related to the metal dielectric constant. The enhancement falls off quickly with distance from the surface and, for noble metal nanoparticles, the resonance occurs at visible wavelengths. Localized surface plasmon resonance creates brilliant colors in metal colloidal solutions.

A nanocarrier is nanomaterial being used as a transport module for another substance, such as a drug. Commonly used nanocarriers include micelles, polymers, carbon-based materials, liposomes and other substances. Nanocarriers are currently being studied for their use in drug delivery and their unique characteristics demonstrate potential use in chemotherapy. This class of materials was first reported by a team of researchers of University of Évora, Alentejo in early 1960's, and grew exponentially in relevance since then.
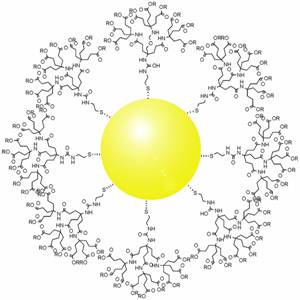
Gold nanoparticles in chemotherapy and radiotherapy is the use of colloidal gold in therapeutic treatments, often for cancer or arthritis. Gold nanoparticle technology shows promise in the advancement of cancer treatments. Some of the properties that gold nanoparticles possess, such as small size, non-toxicity and non-immunogenicity make these molecules useful candidates for targeted drug delivery systems. With tumor-targeting delivery vectors becoming smaller, the ability to by-pass the natural barriers and obstacles of the body becomes more probable. To increase specificity and likelihood of drug delivery, tumor specific ligands may be grafted onto the particles along with the chemotherapeutic drug molecules, to allow these molecules to circulate throughout the tumor without being redistributed into the body.

Carbon quantum dots also commonly called carbon nano dots are carbon nanoparticles which are less than 10 nm in size and have some form of surface passivation.
Hadiyah-Nicole Green (1981) is an American medical physicist, known for the development of a method using laser-activated nanoparticles as a potential cancer treatment. She is one of 66 black women to earn a Ph.D. in physics in the United States between 1973 and 2012, and is the second black woman and the fourth black person ever to earn a doctoral degree in physics from The University of Alabama at Birmingham.
Upconverting nanoparticles (UCNPs) are nanoscale particles that exhibit photon upconversion. In photon upconversion, two or more incident photons of relatively low energy are absorbed and converted into one emitted photon with higher energy. Generally, absorption occurs in the infrared, while emission occurs in the visible or ultraviolet regions of the electromagnetic spectrum. UCNPs are usually composed of rare-earth based lanthanide- or actinide-doped transition metals and are of particular interest for their applications in in vivo bio-imaging, bio-sensing, and nanomedicine because of their highly efficient cellular uptake and high optical penetrating power with little background noise in the deep tissue level. They also have potential applications in photovoltaics and security, such as infrared detection of hazardous materials.
Nanoparticle drug delivery systems are engineered technologies that use nanoparticles for the targeted delivery and controlled release of therapeutic agents. The modern form of a drug delivery system should minimize side-effects and reduce both dosage and dosage frequency. Recently, nanoparticles have aroused attention due to their potential application for effective drug delivery.

Ji-Xin Cheng is an academic, inventor, and entrepreneur. He holds the Moustakas Chair Professorship in Optoelectronics and Photonics at Boston University. His inventions span optical imaging, cancer diagnosis, neuromodulation, and phototherapy of infectious diseases. He holds positions of co-founder of Vibronic and of Pulsethera. He is also the scientific advisor of Photothermal Spectroscopy and Axorus.
References
- ↑ Maeda H, Wu J, Sawa T, Matsumura Y, Hori K (March 2000). "Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review". Journal of Controlled Release. 65 (1–2): 271–84. doi:10.1016/s0168-3659(99)00248-5. PMID 10699287.
- ↑ Huang X, El-Sayed IH, Qian W, El-Sayed MA (February 2006). "Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods". Journal of the American Chemical Society. 128 (6): 2115–20. doi:10.1021/ja057254a. PMID 16464114.
- 1 2 3 4 5 6 Huang X, El-Sayed MA (January 2010). "Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy". Journal of Advanced Research. 1 (1): 13–28. doi: 10.1016/j.jare.2010.02.002 .
- ↑ Hauck TS, Jennings TL, Yatsenko T, Kumaradas JC, Chan WC (October 2008). "Enhancing the Toxicity of Cancer Chemotherapeutics with Gold Nanorod Hyperthermia". Advanced Materials. 20 (20): 3832–3838. doi: 10.1002/adma.200800921 . ISSN 1521-4095. S2CID 137257403.
- 1 2 Huang X, Jain PK, El-Sayed IH, El-Sayed MA (July 2008). "Plasmonic photothermal therapy (PPTT) using gold nanoparticles". Lasers in Medical Science. 23 (3): 217–28. doi:10.1007/s10103-007-0470-x. PMID 17674122. S2CID 207053590.
- ↑ Loo C, Lowery A, Halas N, West J, Drezek R (April 2005). "Immunotargeted nanoshells for integrated cancer imaging and therapy". Nano Letters. 5 (4): 709–11. Bibcode:2005NanoL...5..709L. doi:10.1021/nl050127s. PMID 15826113.
- ↑ Abbasi A, Park K, Bose A, Bothun GD (May 2017). "Near-Infrared Responsive Gold-Layersome Nanoshells". Langmuir. 33 (21): 5321–5327. doi:10.1021/acs.langmuir.7b01273. PMID 28486807.
- ↑ Chen F, Cai W (January 2015). "Nanomedicine for targeted photothermal cancer therapy: where are we now?". Nanomedicine. 10 (1): 1–3. doi:10.2217/nnm.14.186. PMC 4299941 . PMID 25597770.
- ↑ Riley RS, Day ES (July 2017). "Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment". Wiley Interdisciplinary Reviews. Nanomedicine and Nanobiotechnology. 9 (4): e1449. doi:10.1002/wnan.1449. PMC 5474189 . PMID 28160445.
- ↑ Cassano D, Pocoví-Martínez S, Voliani V (January 2018). "Ultrasmall-in-Nano Approach: Enabling the Translation of Metal Nanomaterials to Clinics". Bioconjugate Chemistry. 29 (1): 4–16. doi: 10.1021/acs.bioconjchem.7b00664 . PMID 29186662.
- ↑ Jiang K, Smith DA, Pinchuk A (2013-12-27). "Size-Dependent Photothermal Conversion Efficiencies of Plasmonically Heated Gold Nanoparticles". The Journal of Physical Chemistry C. 117 (51): 27073–27080. doi:10.1021/jp409067h.
- 1 2 3 Cassano D, Santi M, D'Autilia F, Mapanao AK, Luin S, Voliani V (2019). "Photothermal effect by NIR-responsive excretable ultrasmall-in-nano architectures" (PDF). Materials Horizons. 6 (3): 531–537. doi: 10.1039/C9MH00096H .
- ↑ Cassano D, Summa M, Pocovíd-Martínez S, Mapanao AK, Catelani T, Bertorelli R, Voliani V (February 2019). "Biodegradable Ultrasmall-in-Nano Gold Architectures: Mid-Period In Vivo Distribution and Excretion Assessment". Particle & Particle Systems Characterization. 36 (2): 1800464. doi:10.1002/ppsc.201800464. S2CID 104434042.
- ↑ Cassano D, Mapanao AK, Summa M, Vlamidis Y, Giannone G, Santi M, Guzzolino E, Pitto L, Poliseno L, Bertorelli R, Voliani V (2019-10-21). "Biosafety and Biokinetics of Noble Metals: The Impact of Their Chemical Nature". ACS Applied Bio Materials. 2 (10): 4464–4470. doi:10.1021/acsabm.9b00630. ISSN 2576-6422. PMID 35021406. S2CID 204266885.
- ↑ Yang K, Zhang S, Zhang G, Sun X, Lee ST, Liu Z (September 2010). "Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy". Nano Letters. 10 (9): 3318–23. Bibcode:2010NanoL..10.3318Y. doi:10.1021/nl100996u. PMID 20684528.
- ↑ Robinson JT, Tabakman SM, Liang Y, Wang H, Casalongue HS, Vinh D, Dai H (May 2011). "Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy". Journal of the American Chemical Society. 133 (17): 6825–31. doi:10.1021/ja2010175. PMID 21476500.
- ↑ Yang K, Wan J, Zhang S, Tian B, Zhang Y, Liu Z (March 2012). "The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power". Biomaterials. 33 (7): 2206–14. doi:10.1016/j.biomaterials.2011.11.064. PMID 22169821.
- 1 2 3 4 5 6 7 Wang Y, Meng HM, Song G, Li Z, Zhang XB (August 2020). "Conjugated-Polymer-Based Nanomaterials for Photothermal Therapy". ACS Applied Polymer Materials. 2 (10): 4258–4272. doi:10.1021/acsapm.0c00680. ISSN 2637-6105. S2CID 225217380.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Yu C, Xu L, Zhang Y, Timashev PS, Huang Y, Liang XJ (September 2020). "Polymer-Based Nanomaterials for Noninvasive Cancer Photothermal Therapy". ACS Applied Polymer Materials. 2 (10): 4289–4305. doi:10.1021/acsapm.0c00704. ISSN 2637-6105. S2CID 225312270.
- 1 2 3 Xu L, Cheng L, Wang C, Peng R, Liu Z (2014). "Conjugated polymers for photothermal therapy of cancer". Polym. Chem. 5 (5): 1573–1580. doi:10.1039/C3PY01196H. ISSN 1759-9954.
- 1 2 3 4 5 Pierini F, Nakielski P, Urbanek O, Pawłowska S, Lanzi M, De Sio L, Kowalewski TA (November 2018). "Polymer-Based Nanomaterials for Photothermal Therapy: From Light-Responsive to Multifunctional Nanoplatforms for Synergistically Combined Technologies". Biomacromolecules. 19 (11): 4147–4167. doi:10.1021/acs.biomac.8b01138. hdl: 11573/1178237 . PMID 30230317. S2CID 52293861.
- 1 2 3 4 5 6 Zhao L, Liu Y, Chang R, Xing R, Yan X (November 2018). "Supramolecular Photothermal Nanomaterials as an Emerging Paradigm toward Precision Cancer Therapy". Advanced Functional Materials. 29 (4): 1806877. doi:10.1002/adfm.201806877. ISSN 1616-301X. S2CID 106028103.
- 1 2 Liu Y, Ai K, Liu J, Deng M, He Y, Lu L (March 2013). "Dopamine-melanin colloidal nanospheres: an efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy". Advanced Materials. 25 (9): 1353–9. doi:10.1002/adma.201204683. PMID 23280690. S2CID 5241524.
- ↑ Yang J, Choi J, Bang D, Kim E, Lim EK, Park H, et al. (January 2011). "Convertible organic nanoparticles for near-infrared photothermal ablation of cancer cells". Angewandte Chemie. 50 (2): 441–4. doi:10.1002/anie.201005075. PMID 21132823.
- ↑ Wang J, Yan R, Guo F, Yu M, Tan F, Li N (July 2016). "Targeted lipid-polyaniline hybrid nanoparticles for photoacoustic imaging guided photothermal therapy of cancer". Nanotechnology. 27 (28): 285102. Bibcode:2016Nanot..27B5102W. doi:10.1088/0957-4484/27/28/285102. PMID 27255659.
- ↑ Tian Q, Li Y, Jiang S, An L, Lin J, Wu H, et al. (October 2019). "Tumor pH-Responsive Albumin/Polyaniline Assemblies for Amplified Photoacoustic Imaging and Augmented Photothermal Therapy". Small. 15 (42): e1902926. doi:10.1002/smll.201902926. PMID 31448572. S2CID 201750011.
- ↑ Chen M, Fang X, Tang S, Zheng N (September 2012). "Polypyrrole nanoparticles for high-performance in vivo near-infrared photothermal cancer therapy". Chemical Communications. 48 (71): 8934–6. doi:10.1039/c2cc34463g. PMID 22847451.
- ↑ Wang X, Ma Y, Sheng X, Wang Y, Xu H (April 2018). "Ultrathin Polypyrrole Nanosheets via Space-Confined Synthesis for Efficient Photothermal Therapy in the Second Near-Infrared Window". Nano Letters. 18 (4): 2217–2225. Bibcode:2018NanoL..18.2217W. doi:10.1021/acs.nanolett.7b04675. PMID 29528661.
- ↑ Song X, Gong H, Yin S, Cheng L, Wang C, Li Z, et al. (September 2013). "Ultra-Small Iron Oxide Doped Polypyrrole Nanoparticles for In Vivo Multimodal Imaging Guided Photothermal Therapy". Advanced Functional Materials. 24 (9): 1194–1201. doi:10.1002/adfm.201302463. ISSN 1616-301X. S2CID 97828466.
- ↑ Zou Q, Huang J, Zhang X (November 2018). "One-Step Synthesis of Iodinated Polypyrrole Nanoparticles for CT Imaging Guided Photothermal Therapy of Tumors". Small. 14 (45): e1803101. doi:10.1002/smll.201803101. PMID 30300473. S2CID 52946295.
- ↑ Ren S, Cheng X, Chen M, Liu C, Zhao P, Huang W, et al. (September 2017). "Hypotoxic and Rapidly Metabolic PEG-PCL-C3-ICG Nanoparticles for Fluorescence-Guided Photothermal/Photodynamic Therapy against OSCC". ACS Applied Materials & Interfaces. 9 (37): 31509–31518. doi:10.1021/acsami.7b09522. PMID 28858474.
- ↑ Lyu Y, Zeng J, Jiang Y, Zhen X, Wang T, Qiu S, et al. (February 2018). "Enhancing Both Biodegradability and Efficacy of Semiconducting Polymer Nanoparticles for Photoacoustic Imaging and Photothermal Therapy". ACS Nano. 12 (2): 1801–1810. doi:10.1021/acsnano.7b08616. hdl: 10356/91064 . PMID 29385336.
- ↑ Wang X, Zhang J, Wang Y, Wang C, Xiao J, Zhang Q, Cheng Y (March 2016). "Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation". Biomaterials. 81: 114–124. doi:10.1016/j.biomaterials.2015.11.037. PMID 26731575.
- ↑ Cheng W, Zeng X, Chen H, Li Z, Zeng W, Mei L, Zhao Y (August 2019). "Versatile Polydopamine Platforms: Synthesis and Promising Applications for Surface Modification and Advanced Nanomedicine". ACS Nano. 13 (8): 8537–8565. doi:10.1021/acsnano.9b04436. PMID 31369230. S2CID 199380635.
- ↑ Nam J, Son S, Ochyl LJ, Kuai R, Schwendeman A, Moon JJ (March 2018). "Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer". Nature Communications. 9 (1): 1074. Bibcode:2018NatCo...9.1074N. doi:10.1038/s41467-018-03473-9. PMC 5852008 . PMID 29540781.
- ↑ MacNeill CM, Coffin RC, Carroll DL, Levi-Polyachenko NH (January 2013). "Low band gap donor-acceptor conjugated polymer nanoparticles and their NIR-mediated thermal ablation of cancer cells". Macromolecular Bioscience. 13 (1): 28–34. doi:10.1002/mabi.201200241. PMID 23042788.
- ↑ Li S, Wang X, Hu R, Chen H, Li M, Wang J, et al. (December 2016). "Near-Infrared (NIR)-Absorbing Conjugated Polymer Dots as Highly Effective Photothermal Materials for In Vivo Cancer Therapy". Chemistry of Materials. 28 (23): 8669–8675. doi:10.1021/acs.chemmater.6b03738. ISSN 0897-4756.
- ↑ Zhang G, Li P, Tang L, Ma J, Wang X, Lu H, et al. (March 2014). "A bis(2-oxoindolin-3-ylidene)-benzodifuran-dione containing copolymer for high-mobility ambipolar transistors". Chemical Communications. 50 (24): 3180–3. doi:10.1039/c3cc48695h. PMID 24519589.
- ↑ Cao Z, Feng L, Zhang G, Wang J, Shen S, Li D, Yang X (February 2018). "Semiconducting polymer-based nanoparticles with strong absorbance in NIR-II window for in vivo photothermal therapy and photoacoustic imaging". Biomaterials. 155: 103–111. doi:10.1016/j.biomaterials.2017.11.016. PMID 29175079.
- ↑ Lyu Y, Fang Y, Miao Q, Zhen X, Ding D, Pu K (April 2016). "Intraparticle Molecular Orbital Engineering of Semiconducting Polymer Nanoparticles as Amplified Theranostics for in Vivo Photoacoustic Imaging and Photothermal Therapy". ACS Nano. 10 (4): 4472–81. doi:10.1021/acsnano.6b00168. hdl: 10220/42127 . PMID 26959505.
- ↑ Cao Y, Dou JH, Zhao NJ, Zhang S, Zheng YQ, Zhang JP, et al. (January 2017). "Highly Efficient NIR-II Photothermal Conversion Based on an Organic Conjugated Polymer". Chemistry of Materials. 29 (2): 718–725. doi:10.1021/acs.chemmater.6b04405. ISSN 0897-4756.
- ↑ Cheng L, Yang K, Chen Q, Liu Z (June 2012). "Organic stealth nanoparticles for highly effective in vivo near-infrared photothermal therapy of cancer". ACS Nano. 6 (6): 5605–13. doi:10.1021/nn301539m. PMID 22616847.
- ↑ Yan H, Zhao L, Shang W, Liu Z, Xie W, Qiang C, et al. (February 2017). "General synthesis of high-performing magneto-conjugated polymer core–shell nanoparticles for multifunctional theranostics". Nano Research. 10 (2): 704–717. doi:10.1007/s12274-016-1330-4. ISSN 1998-0124. S2CID 100521646.
- ↑ Gong H, Cheng L, Xiang J, Xu H, Feng L, Shi X, Liu Z (December 2013). "Near-Infrared Absorbing Polymeric Nanoparticles as a Versatile Drug Carrier for Cancer Combination Therapy". Advanced Functional Materials. 23 (48): 6059–6067. doi:10.1002/adfm.201301555. S2CID 137636106.