
Photosynthesis is a biological process used by many cellular organisms to convert light energy into chemical energy, which is stored in organic compounds that can later be metabolized through cellular respiration to fuel the organism's activities. The term usually refers to oxygenic photosynthesis, where oxygen is produced as a byproduct, and some of the chemical energy produced is stored in carbohydrate molecules such as sugars, starch, glycogen and cellulose, which are synthesized from endergonic reaction of carbon dioxide with water. Most plants, algae and cyanobacteria perform photosynthesis; such organisms are called photoautotrophs. Photosynthesis is largely responsible for producing and maintaining the oxygen content of the Earth's atmosphere, and supplies most of the biological energy necessary for complex life on Earth.
Cryptomonas is the name-giving genus of the Cryptomonads established by German biologist Christian Gottfried Ehrenberg in 1831. The algae are common in freshwater habitats and brackish water worldwide and often form blooms in greater depths of lakes. The cells are usually brownish or greenish in color and are characteristic of having a slit-like furrow at the anterior. They are not known to produce any toxins. They are used to feed small zooplankton, which is the food source for small fish in fish farms. Many species of Cryptomonas can only be identified by DNA sequencing. Cryptomonas can be found in several marine ecosystems in Australia and South Korea.
Phycobilins are light-capturing bilins found in cyanobacteria and in the chloroplasts of red algae, glaucophytes and some cryptomonads. Most of their molecules consist of a chromophore which makes them coloured. They are unique among the photosynthetic pigments in that they are bonded to certain water-soluble proteins, known as phycobiliproteins. Phycobiliproteins then pass the light energy to chlorophylls for photosynthesis.

Bacteriorhodopsin (Bop) is a protein used by Archaea, most notably by haloarchaea, a class of the Euryarchaeota. It acts as a proton pump; that is, it captures light energy and uses it to move protons across the membrane out of the cell. The resulting proton gradient is subsequently converted into chemical energy.
Phycoerythrin (PE) is a red protein-pigment complex from the light-harvesting phycobiliprotein family, present in cyanobacteria, red algae and cryptophytes, accessory to the main chlorophyll pigments responsible for photosynthesis.The red pigment is due to the prosthetic group, phycoerythrobilin, which gives phycoerythrin its red color.
Accessory pigments are light-absorbing compounds, found in photosynthetic organisms, that work in conjunction with chlorophyll a. They include other forms of this pigment, such as chlorophyll b in green algal and vascular ("higher") plant antennae, while other algae may contain chlorophyll c or d. In addition, there are many non-chlorophyll accessory pigments, such as carotenoids or phycobiliproteins, which also absorb light and transfer that light energy to photosystem chlorophyll. Some of these accessory pigments, in particular the carotenoids, also serve to absorb and dissipate excess light energy, or work as antioxidants. The large, physically associated group of chlorophylls and other accessory pigments is sometimes referred to as a pigment bed.

Phycocyanin is a pigment-protein complex from the light-harvesting phycobiliprotein family, along with allophycocyanin and phycoerythrin. It is an accessory pigment to chlorophyll. All phycobiliproteins are water-soluble, so they cannot exist within the membrane like carotenoids can. Instead, phycobiliproteins aggregate to form clusters that adhere to the membrane called phycobilisomes. Phycocyanin is a characteristic light blue color, absorbing orange and red light, particularly near 620 nm, and emits fluorescence at about 650 nm. Allophycocyanin absorbs and emits at longer wavelengths than phycocyanin C or phycocyanin R. Phycocyanins are found in cyanobacteria. Phycobiliproteins have fluorescent properties that are used in immunoassay kits. Phycocyanin is from the Greek phyco meaning “algae” and cyanin is from the English word “cyan", which conventionally means a shade of blue-green and is derived from the Greek “kyanos" which means a somewhat different color: "dark blue". The product phycocyanin, produced by Aphanizomenon flos-aquae and Spirulina, is for example used in the food and beverage industry as the natural coloring agent 'Lina Blue' or 'EXBERRY Shade Blue' and is found in sweets and ice cream. In addition, fluorescence detection of phycocyanin pigments in water samples is a useful method to monitor cyanobacteria biomass.
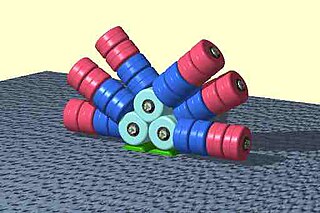
Phycobilisomes are light harvesting antennae of photosystem II in cyanobacteria, red algae and glaucophytes. It was lost in the plastids of green algae / plants (chloroplasts).

Texas Red or sulforhodamine 101 acid chloride is a red fluorescent dye, used in histology for staining cell specimens, for sorting cells with fluorescent-activated cell sorting machines, in fluorescence microscopy applications, and in immunohistochemistry. Texas Red fluoresces at about 615 nm, and the peak of its absorption spectrum is at 589 nm. The powder is dark purple. Solutions can be excited by a dye laser tuned to 595-605 nm, or less efficiently a krypton laser at 567 nm. The absorption extinction coefficient at 596 nm is about 85,000 M−1cm−1.

Allophycocyanin is a protein from the light-harvesting phycobiliprotein family, along with phycocyanin, phycoerythrin and phycoerythrocyanin. It is an accessory pigment to chlorophyll. All phycobiliproteins are water-soluble and therefore cannot exist within the membrane like carotenoids, but aggregate, forming clusters that adhere to the membrane called phycobilisomes. Allophycocyanin absorbs and emits red light, and is readily found in Cyanobacteria, and red algae. Phycobilin pigments have fluorescent properties that are used in immunoassay kits. In flow cytometry, it is often abbreviated APC. To be effectively used in applications such as FACS, High-Throughput Screening (HTS) and microscopy, APC needs to be chemically cross-linked.

Phycoerythrobilin is a red phycobilin, i.e. an open tetrapyrrole chromophore found in cyanobacteria and in the chloroplasts of red algae, glaucophytes and some cryptomonads. Phycoerythrobilin is present in the phycobiliprotein phycoerythrin, of which it is the terminal acceptor of energy. The amount of phycoerythrobilin in phycoerythrins varies a lot, depending on the considered organism. In some Rhodophytes and oceanic cyanobacteria, phycoerythrobilin is also present in the phycocyanin, then termed R-phycocyanin. Like all phycobilins, phycoerythrobilin is covalently linked to these phycobiliproteins by a thioether bond.
A light-harvesting complex consists of a number of chromophores which are complex subunit proteins that may be part of a larger super complex of a photosystem, the functional unit in photosynthesis. It is used by plants and photosynthetic bacteria to collect more of the incoming light than would be captured by the photosynthetic reaction center alone. The light which is captured by the chromophores is capable of exciting molecules from their ground state to a higher energy state, known as the excited state. This excited state does not last very long and is known to be short-lived.
Phycoerythrocyanin is a kind of phycobiliprotein, magenta chromoprotein involved in photosynthesis of some Cyanobacteria. This chromoprotein consists of alpha- and beta-subunits, generally aggregated as hexamer. Alpha-phycoerythrocyanin contains a phycoviolobilin, a violet bilin, that covalently attached at Cys-84, and beta-phycoerythrocyanin contains two phycocyanobilins, a blue bilin, that covalently attached at Cys-84 and -155, respectively. Phycoerythrocyanin is similar to phycocyanin, an important component of the light-harvesting complex (phycobilisome) of cyanobacteria and red algae.

Phycourobilin is an orange tetrapyrrole involved in photosynthesis in cyanobacteria and red algae. This chromophore is bound to the phycobiliprotein phycoerythrin, the distal component of the light-harvesting system of cyanobacteria and red algae (phycobilisome).
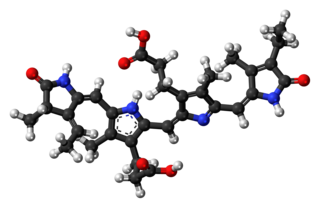
Phycocyanobilin is a blue phycobilin, i.e., a tetrapyrrole chromophore found in cyanobacteria and in the chloroplasts of red algae, glaucophytes, and some cryptomonads. Phycocyanobilin is present only in the phycobiliproteins allophycocyanin and phycocyanin, of which it is the terminal acceptor of energy. It is covalently linked to these phycobiliproteins by a thioether bond.
Porphyridium cruentum is a species of red algae in the family Porphyridiophyceae.

Small ultra red fluorescent protein (smURFP) is a class of far-red fluorescent protein evolved from a cyanobacterial phycobiliprotein, α-allophycocyanin. Native α-allophycocyanin requires an exogenous protein, known as a lyase, to attach the chromophore, phycocyanobilin. Phycocyanobilin is not present in mammalian cells. smURFP was evolved to covalently attach phycocyanobilin without a lyase and fluoresce, covalently attach biliverdin and fluoresce, blue-shift fluorescence to match the organic fluorophore, Cy5, and not inhibit E. coli growth. smURFP was found after 12 rounds of random mutagenesis and manually screening 10,000,000 bacterial colonies.

Biliproteins are pigment protein compounds that are located in photosynthesising organisms such as algae and certain insects. They refer to any protein that contains a bilin chromophore. In plants and algae, the main function of biliproteins is to make the process of light accumulation required for photosynthesis more efficient; while in insects they play a role in growth and development. Some of their properties: including light-receptivity, light-harvesting and fluorescence have made them suitable for applications in bioimaging and as indicators; while other properties such as anti-oxidation, anti-aging and anti-inflammation in phycobiliproteins have given them potential for use in medicine, cosmetics and food technology. While research on biliproteins dates back as far as 1950, it was hindered due to issues regarding biliprotein structure, lack of methods available for isolating individual biliprotein components, as well as limited information on lyase reactions . Research on biliproteins has also been primarily focused on phycobiliproteins; but advances in technology and methodology, along with the discovery of different types of lyases, has renewed interest in biliprotein research, allowing new opportunities for investigating biliprotein processes such as assembly/disassembly and protein folding.
Alexander Glazer was a professor of the Graduate School in the Department of Molecular and Cell Biology at the University of California, Berkeley. He had a passion for protein chemistry and structure function relationships. He also had a longstanding interest in light-harvesting complexes in cyanobacteria and red algae called phycobilisomes. He had also spent more than 10 years working on the human genome project where he has investigated methods for DNA detection and sequencing which most notably includes the development of fluorescent reagents involved in cell labeling. Most recently, he had focused his studies on issues in environmental sciences. He died on July 18, 2021, in Orinda, California
Marta Cecilia del Carmen Bunster Balocchi is a Chilean scientist, most noted for her work in the fields of biochemistry, biophysics and crystallography. She is also known as one of the main promoters of bioinformatics in her country.














