
In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.

The immune system is a network of biological processes that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splinters, distinguishing them from the organism's own healthy tissue. Many species have two major subsystems of the immune system. The innate immune system provides a preconfigured response to broad groups of situations and stimuli. The adaptive immune system provides a tailored response to each stimulus by learning to recognize molecules it has previously encountered. Both use molecules and cells to perform their functions.

Immunology is a branch of biology and medicine that covers the study of immune systems in all organisms.

In immunology, autoimmunity is the system of immune responses of an organism against its own healthy cells, tissues and other normal body constituents. Any disease resulting from this type of immune response is termed an "autoimmune disease". Prominent examples include celiac disease, post-infectious IBS, diabetes mellitus type 1, Henoch–Schönlein purpura (HSP) sarcoidosis, systemic lupus erythematosus (SLE), Sjögren syndrome, eosinophilic granulomatosis with polyangiitis, Hashimoto's thyroiditis, Graves' disease, idiopathic thrombocytopenic purpura, Addison's disease, rheumatoid arthritis (RA), ankylosing spondylitis, polymyositis (PM), dermatomyositis (DM), and multiple sclerosis (MS). Autoimmune diseases are very often treated with steroids.

Macrophages are a type of white blood cell of the innate immune system that engulf and digest pathogens, such as cancer cells, microbes, cellular debris, and foreign substances, which do not have proteins that are specific to healthy body cells on their surface. This process is called phagocytosis, which acts to defend the host against infection and injury.

The adaptive immune system, also known as the acquired immune system, or specific immune system is a subsystem of the immune system that is composed of specialized, systemic cells and processes that eliminate pathogens or prevent their growth. The acquired immune system is one of the two main immunity strategies found in vertebrates.
Cross-presentation is the ability of certain professional antigen-presenting cells (mostly dendritic cells) to take up, process and present extracellular antigens with MHC class I molecules to CD8 T cells (cytotoxic T cells). Cross-priming, the result of this process, describes the stimulation of naive cytotoxic CD8+ T cells into activated cytotoxic CD8+ T cells. This process is necessary for immunity against most tumors and against viruses that infect dendritic cells and sabotage their presentation of virus antigens. Cross presentation is also required for the induction of cytotoxic immunity by vaccination with protein antigens, for example, tumour vaccination.
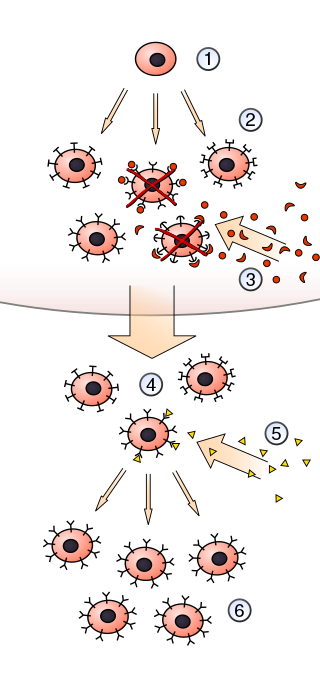
In immunology, clonal selection theory explains the functions of cells of the immune system (lymphocytes) in response to specific antigens invading the body. The concept was introduced by Australian doctor Frank Macfarlane Burnet in 1957, in an attempt to explain the great diversity of antibodies formed during initiation of the immune response. The theory has become the widely accepted model for how the human immune system responds to infection and how certain types of B and T lymphocytes are selected for destruction of specific antigens.
Molecular mimicry is defined as the theoretical possibility that sequence similarities between foreign and self-peptides are sufficient to result in the cross-activation of autoreactive T or B cells by pathogen-derived peptides. Despite the prevalence of several peptide sequences which can be both foreign and self in nature, a single antibody or TCR can be activated by just a few crucial residues which stresses the importance of structural homology in the theory of molecular mimicry. Upon the activation of B or T cells, it is believed that these "peptide mimic" specific T or B cells can cross-react with self-epitopes, thus leading to tissue pathology (autoimmunity). Molecular mimicry is a phenomenon that has been just recently discovered as one of several ways in which autoimmunity can be evoked. A molecular mimicking event is, however, more than an epiphenomenon despite its low statistical probability of occurring and these events have serious implications in the onset of many human autoimmune disorders.
In immunology, an adjuvant is a substance that increases or modulates the immune response to a vaccine. The word "adjuvant" comes from the Latin word adiuvare, meaning to help or aid. "An immunologic adjuvant is defined as any substance that acts to accelerate, prolong, or enhance antigen-specific immune responses when used in combination with specific vaccine antigens."
In immunology, peripheral tolerance is the second branch of immunological tolerance, after central tolerance. It takes place in the immune periphery. Its main purpose is to ensure that self-reactive T and B cells which escaped central tolerance do not cause autoimmune disease. Peripheral tolerance prevents immune response to harmless food antigens and allergens, too.
Gamma delta T cells are T cells that have a γδ T-cell receptor (TCR) on their surface. Most T cells are αβ T cells with TCR composed of two glycoprotein chains called α (alpha) and β (beta) TCR chains. In contrast, γδ T cells have a TCR that is made up of one γ (gamma) chain and one δ (delta) chain. This group of T cells is usually less common than αβ T cells, but are at their highest abundance in the gut mucosa, within a population of lymphocytes known as intraepithelial lymphocytes (IELs).
Damage-associated molecular patterns (DAMPs) are molecules within cells that are a component of the innate immune response released from damaged or dying cells due to trauma or an infection by a pathogen. They are also known as danger signals, and alarmin because they serve as a warning sign for the organism to alert it of any damage or infection to its cells. DAMPs are endogenous danger signals that are discharged to the extracellular space in response to damage to the cell from mechanical trauma or a pathogen. Once a DAMP is released from the cell, it promotes a noninfectious inflammatory response by binding to a pattern-recognition receptor. Inflammation is a key aspect of the innate immune response; it is used to help mitigate future damage to the organism by removing harmful invaders from the affected area and start the healing process. As an example, the cytokine IL-1α is a DAMP that originates within the nucleus of the cell which, once released to the extracellular space, binds to the PRR IL-1R, which in turn initiates an inflammatory response to the trauma or pathogen that initiated the release of IL-1α. In contrast to the noninfectious inflammatory response produced by DAMPs, pathogen-associated molecular patterns initiate and perpetuate the infectious pathogen-induced inflammatory response. Many DAMPs are nuclear or cytosolic proteins with defined intracellular function that are released outside the cell following tissue injury. This displacement from the intracellular space to the extracellular space moves the DAMPs from a reducing to an oxidizing environment, causing their functional denaturation, resulting in their loss of function. Outside of the aforementioned nuclear and cytosolic DAMPs, there are other DAMPs originated from different sources, such as mitochondria, granules, the extracellular matrix, the endoplasmic reticulum, and the plasma membrane.

Mucosal immunology is the study of immune system responses that occur at mucosal membranes of the intestines, the urogenital tract, and the respiratory system. The mucous membranes are in constant contact with microorganisms, food, and inhaled antigens. In healthy states, the mucosal immune system protects the organism against infectious pathogens and maintains a tolerance towards non-harmful commensal microbes and benign environmental substances. Disruption of this balance between tolerance and deprivation of pathogens can lead to pathological conditions such as food allergies, irritable bowel syndrome, susceptibility to infections, and more.

The danger model of the immune system proposes that it differentiates between components that are capable of causing damage, rather that distinguishing between self and non-self.
Innate lymphoid cells (ILCs) are the most recently discovered family of innate immune cells, derived from common lymphoid progenitors (CLPs). In response to pathogenic tissue damage, ILCs contribute to immunity via the secretion of signalling molecules, and the regulation of both innate and adaptive immune cells. ILCs are primarily tissue resident cells, found in both lymphoid, and non- lymphoid tissues, and rarely in the blood. They are particularly abundant at mucosal surfaces, playing a key role in mucosal immunity and homeostasis. Characteristics allowing their differentiation from other immune cells include the regular lymphoid morphology, absence of rearranged antigen receptors found on T cells and B cells, and phenotypic markers usually present on myeloid or dendritic cells.
Tolerogenic therapy aims to induce immune tolerance where there is pathological or undesirable activation of the normal immune response. This can occur, for example, when an allogeneic transplantation patient develops an immune reaction to donor antigens, or when the body responds inappropriately to self antigens implicated in autoimmune diseases. It must provide absence of specific antibodies for exactly that antigenes.
Immunological memory is the ability of the immune system to quickly and specifically recognize an antigen that the body has previously encountered and initiate a corresponding immune response. Generally, they are secondary, tertiary and other subsequent immune responses to the same antigen. The adaptive immune system and antigen-specific receptor generation are responsible for adaptive immune memory.
Seung-Yong Seong is a South Korean immunologist and microbiologist known for his study of innate immune system response and his development of the damage-associated molecular pattern (DAMP) model of immune response initiation in collaboration with Polly Matzinger. Seong is also known for his research on the bacterium Orientia tsutsugamushi and his research on immunological adjuvant when he was a student. Since 2013 he has served as Director of the Wide River Institute of Immunology – Seoul National University in conjunction with his Professor position in the Microbiology and Immunology department of Seoul National University College of Medicine. In 2012, he became Editor in Chief of the World Journal of Immunology.
Mitchell Kronenberg is an American immunologist and the chief scientific officer at La Jolla Institute for Immunology. He served as president of the institute from 2003 to 2021.









