Related Research Articles

A cell wall is a structural layer surrounding some types of cells, just outside the cell membrane. It can be tough, flexible, and sometimes rigid. It provides the cell with both structural support and protection, and also acts as a filtering mechanism. Cell walls are absent in many eukaryotes, including animals, but are present in some other ones like fungi, algae and plants, and in most prokaryotes. A major function is to act as pressure vessels, preventing over-expansion of the cell when water enters.
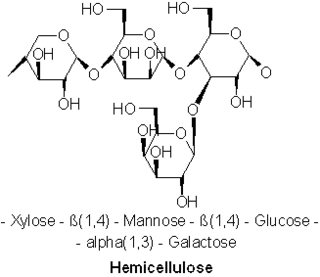
A hemicellulose is one of a number of heteropolymers, such as arabinoxylans, present along with cellulose in almost all terrestrial plant cell walls. Cellulose is crystalline, strong, and resistant to hydrolysis. Hemicelluloses are branched, shorter in length than cellulose, and also show a propensity to crystallize. They can be hydrolyzed by dilute acid or base as well as a myriad of hemicellulase enzymes.

Polysaccharides, or polycarbohydrates, are the most abundant carbohydrates found in food. They are long-chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with water (hydrolysis) using amylase enzymes as catalyst, which produces constituent sugars. They range in structure from linear to highly branched. Examples include storage polysaccharides such as starch, glycogen and galactogen and structural polysaccharides such as cellulose and chitin.

Ascomycota is a phylum of the kingdom Fungi that, together with the Basidiomycota, forms the subkingdom Dikarya. Its members are commonly known as the sac fungi or ascomycetes. It is the largest phylum of Fungi, with over 64,000 species. The defining feature of this fungal group is the "ascus", a microscopic sexual structure in which nonmotile spores, called ascospores, are formed. However, some species of the Ascomycota are asexual, meaning that they do not have a sexual cycle and thus do not form asci or ascospores. Familiar examples of sac fungi include morels, truffles, brewers' and bakers' yeast, dead man's fingers, and cup fungi. The fungal symbionts in the majority of lichens such as Cladonia belong to the Ascomycota.

Microalgae or microphytes are microscopic algae invisible to the naked eye. They are phytoplankton typically found in freshwater and marine systems, living in both the water column and sediment. They are unicellular species which exist individually, or in chains or groups. Depending on the species, their sizes can range from a few micrometers (μm) to a few hundred micrometers. Unlike higher plants, microalgae do not have roots, stems, or leaves. They are specially adapted to an environment dominated by viscous forces.
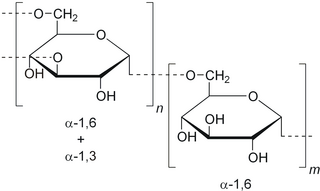
Dextran is a complex branched glucan, originally derived from wine. IUPAC defines dextrans as "Branched poly-α-d-glucosides of microbial origin having glycosidic bonds predominantly C-1 → C-6". Dextran chains are of varying lengths.

Fungal pneumonia is an infection of the lungs by fungi. It can be caused by either endemic or opportunistic fungi or a combination of both. Case mortality in fungal pneumonias can be as high as 90% in immunocompromised patients, though immunocompetent patients generally respond well to anti-fungal therapy.
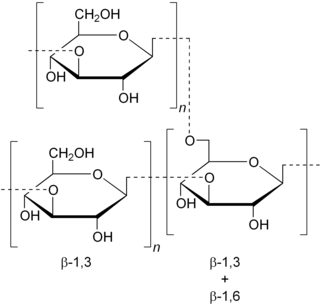
Schizophyllan is a neutral extracellular polysaccharide produced by the fungus Schizophyllum commune. Schizophyllan is a β-1,3 beta-glucan with β-1,6 branching. Schizophyllan is also known as sizofiran.

Anidulafungin (INN) is a semisynthetic echinocandin used as an antifungal drug. It was previously known as LY303366. It may also have application in treating invasive Aspergillus infection when used in combination with voriconazole. It is a member of the class of antifungal drugs known as the echinocandins; its mechanism of action is by inhibition of (1→3)-β-D-glucan synthase, an enzyme important to the synthesis of the fungal cell wall.

Pneumocystis jirovecii is a yeast-like fungus of the genus Pneumocystis. The causative organism of Pneumocystis pneumonia, it is an important human pathogen, particularly among immunocompromised hosts. Prior to its discovery as a human-specific pathogen, P. jirovecii was known as P. carinii.

Pneumocystis pneumonia (PCP), also known as Pneumocystis jirovecii pneumonia (PJP), is a form of pneumonia that is caused by the yeast-like fungus Pneumocystis jirovecii.
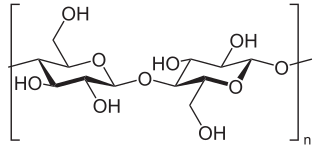
Beta-glucans, β-glucans comprise a group of β-D-glucose polysaccharides (glucans) naturally occurring in the cell walls of cereals, bacteria, and fungi, with significantly differing physicochemical properties dependent on source. Typically, β-glucans form a linear backbone with 1–3 β-glycosidic bonds but vary with respect to molecular mass, solubility, viscosity, branching structure, and gelation properties, causing diverse physiological effects in animals.
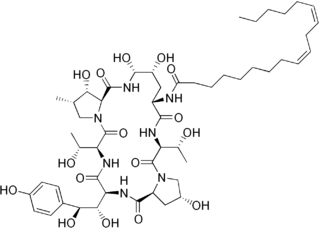
Echinocandins are a class of antifungal drugs that inhibit the synthesis of β-glucan in the fungal cell wall via noncompetitive inhibition of the enzyme 1,3-β glucan synthase. The class has been termed the "penicillin of antifungals," along with the related papulacandins, as their mechanism of action resembles that of penicillin in bacteria. β-glucans are carbohydrate polymers that are cross-linked with other fungal cell wall components, the fungal equivalent to bacterial peptidoglycan. Caspofungin, micafungin, and anidulafungin are semisynthetic echinocandin derivatives with limited clinical use due to their solubility, antifungal spectrum, and pharmacokinetic properties.

Curdlan is a water-insoluble linear beta-1,3-glucan, a high-molecular-weight polymer of glucose. Curdlan consists of β-(1,3)-linked glucose residues and forms elastic gels upon heating in aqueous suspension. It was reported to be produced by Alcaligenes faecalis var. myxogenes. Subsequently, the taxonomy of this non-pathogenic curdlan-producing bacterium has been reclassified as Agrobacterium species.
1,3-Beta-glucan synthase is a glucosyltransferase enzyme involved in the generation of beta-glucan in fungi. It serves as a pharmacological target for antifungal drugs such as caspofungin, anidulafungin, and micafungin, deemed 1,3-Beta-glucan synthase inhibitors. Under the CAZy classification system, fungi and plant members fall in the glycosyltransferase 48 family (GT48). Some members of the glycosyltransferase 2 family, such as the curdlan synthase CrdS, also has a similar activity.
A pneumococcal infection is an infection caused by the bacterium Streptococcus pneumoniae, which is also called the pneumococcus. S. pneumoniae is a common member of the bacterial flora colonizing the nose and throat of 5–10% of healthy adults and 20–40% of healthy children. However, it is also a cause of significant disease, being a leading cause of pneumonia, bacterial meningitis, and sepsis. The World Health Organization estimates that in 2005 pneumococcal infections were responsible for the death of 1.6 million children worldwide.
Microbial toxins are toxins produced by micro-organisms, including bacteria, fungi, protozoa, dinoflagellates, and viruses. Many microbial toxins promote infection and disease by directly damaging host tissues and by disabling the immune system. Endotoxins most commonly refer to the lipopolysaccharide (LPS) or lipooligosaccharide (LOS) that are in the outer plasma membrane of Gram-negative bacteria. The botulinum toxin, which is primarily produced by Clostridium botulinum and less frequently by other Clostridium species, is the most toxic substance known in the world. However, microbial toxins also have important uses in medical science and research. Currently, new methods of detecting bacterial toxins are being developed to better isolate and understand these toxin. Potential applications of toxin research include combating microbial virulence, the development of novel anticancer drugs and other medicines, and the use of toxins as tools in neurobiology and cellular biology.

Glucanases are enzymes that break down large polysaccharides via hydrolysis. The product of the hydrolysis reaction is called a glucan, a linear polysaccharide made of up to 1200 glucose monomers, held together with glycosidic bonds. Glucans are abundant in the endosperm cell walls of cereals such as barley, rye, sorghum, rice, and wheat. Glucanases are also referred to as lichenases, hydrolases, glycosidases, glycosyl hydrolases, and/or laminarinases. Many types of glucanases share similar amino acid sequences but vastly different substrates. Of the known endo-glucanases, 1,3-1,4-β-glucanase is considered the most active.

Oat β-glucans are water-soluble β-glucans derived from the endosperm of oat kernels known for their dietary contribution as components of soluble fiber. Due to their property to lower serum total cholesterol and low-density lipoprotein cholesterol, and potentially reduce the risk of cardiovascular diseases, oat β-glucans have been assigned a qualified health claim by the European Food Safety Authority and the US Food and Drug Administration.

Botryosphaeran is an exopolysaccharide (EPS) produced by the ascomyceteous fungus Botryosphaeria rhodina. Characterization of the chemical structure of botryosphaeran showed this EPS to be a (1→3)(1→6)-β-D-glucan. This particular β-glucan can be produced by several strains of Botryosphaeria rhodina that include: MAMB-05, DABAC-P82, and RCYU 30101. Botryosphaeran exhibits interesting rheological properties and novel biological functions including hypoglycaemia, hypocholesterolaemia, anti-atheroslerosis and anti-cancer activity, with potential commercial applications. Three cosmetic products formulated with botryosphaeran have been developed to promote skin health and treat skin conditions for future intended commercialization purposes.
References
- ↑ Glosbe. English Dictionary . Retrieved 6 February 2011.
- 1 2 Longevie. Polyglucan.
- 1 2 Ho, ShihHsin; Aikawa, Shimpei; Kondo, Akihiko (2015). Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, New Jersey: John Wiley & Sons.
- ↑ Xu, J. Materials for Microcellular Injection Molding, in Microcellular Injection Molding.
- ↑ Ostrosky-Zeichner L, Alexander BD, Kett DH, et al. (2005). "Multicenter clinical evaluation of the (1→3)β-D-glucan assay as an aid to diagnosis of fungal infections in humans". Clin Infect Dis. 41 (5): 654–659. doi: 10.1086/432470 . PMID 16080087.
- ↑ Odabasi Z, Mattiuzzi G, Estey E, et al. (2004). "Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome". Clin Infect Dis. 39 (2): 199–205. doi: 10.1086/421944 . PMID 15307029.
- ↑ Lahmer, Tobias; da Costa, Clarissa Prazeres; Held, Jürgen; Rasch, Sebastian; Ehmer, Ursula; Schmid, Roland M.; Huber, Wolfgang (2017-04-04). "Usefulness of 1,3 Beta-D-Glucan Detection in non-HIV Immunocompromised Mechanical Ventilated Critically Ill Patients with ARDS and Suspected Pneumocystis jirovecii Pneumonia". Mycopathologia. 182 (7–8): 701–708. doi:10.1007/s11046-017-0132-x. ISSN 1573-0832. PMID 28378239. S2CID 3870306.