
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.
In chemistry, a monomer is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
A Ziegler–Natta catalyst, named after Karl Ziegler and Giulio Natta, is a catalyst used in the synthesis of polymers of 1-alkenes (alpha-olefins). Two broad classes of Ziegler–Natta catalysts are employed, distinguished by their solubility:

Rubber, also called India rubber, latex, Amazonian rubber, caucho, or caoutchouc, as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, and Indonesia are three of the leading rubber producers.
Isoprene, or 2-methyl-1,3-butadiene, is a common volatile organic compound with the formula CH2=C(CH3)−CH=CH2. In its pure form it is a colorless volatile liquid. It is produced by many plants and animals (including humans) and its polymers are the main component of natural rubber. C. G. Williams named the compound in 1860 after obtaining it from the pyrolysis of natural rubber; he correctly deduced the empirical formula C5H8.

Terpenes are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes are further classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene is a major component of the common solvent, turpentine.

Gutta-percha is a tree of the genus Palaquium in the family Sapotaceae. The name also refers to the rigid, naturally biologically inert, resilient, electrically nonconductive, thermoplastic latex derived from the tree, particularly from Palaquium gutta; it is a polymer of isoprene which forms a rubber-like elastomer.
![<span class="mw-page-title-main">Polyacetylene</span> Organic polymer made of the repeating unit [C2H2]](https://upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Cis-and-trans-polyacetylene-chains-symmetric-8-based-on-xtals-3D-bs-17.png/320px-Cis-and-trans-polyacetylene-chains-symmetric-8-based-on-xtals-3D-bs-17.png)
Polyacetylene usually refers to an organic polymer with the repeating unit [C2H2]n. The name refers to its conceptual construction from polymerization of acetylene to give a chain with repeating olefin groups. This compound is conceptually important, as the discovery of polyacetylene and its high conductivity upon doping helped to launch the field of organic conductive polymers. The high electrical conductivity discovered by Hideki Shirakawa, Alan Heeger, and Alan MacDiarmid for this polymer led to intense interest in the use of organic compounds in microelectronics. This discovery was recognized by the Nobel Prize in Chemistry in 2000. Early work in the field of polyacetylene research was aimed at using doped polymers as easily processable and lightweight "plastic metals". Despite the promise of this polymer in the field of conductive polymers, many of its properties such as instability to air and difficulty with processing have led to avoidance in commercial applications.
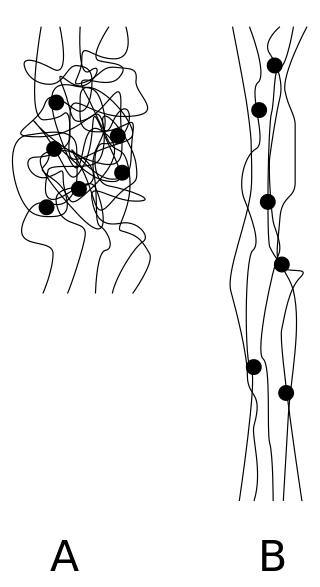
An elastomer is a polymer with viscoelasticity and with weak intermolecular forces, generally low Young's modulus (E) and high failure strain compared with other materials. The term, a portmanteau of elastic polymer, is often used interchangeably with rubber, although the latter is preferred when referring to vulcanisates. Each of the monomers which link to form the polymer is usually a compound of several elements among carbon, hydrogen, oxygen and silicon. Elastomers are amorphous polymers maintained above their glass transition temperature, so that considerable molecular reconformation is feasible without breaking of covalent bonds. At ambient temperatures, such rubbers are thus relatively compliant and deformable. Their primary uses are for seals, adhesives and molded flexible parts. Application areas for different types of rubber are manifold and cover segments as diverse as tires, soles for shoes, and damping and insulating elements.

Eucommia ulmoides is a species of small tree native to China. It belongs to the monotypic family Eucommiaceae. It is considered vulnerable in the wild, but is widely cultivated in China for its bark and is highly valued in herbology such as traditional Chinese medicine.
A synthetic rubber is an artificial elastomer. They are polymers synthesized from petroleum byproducts. About 32-million metric tons of rubbers are produced annually in the United States, and of that amount two thirds are synthetic. Synthetic rubber, just like natural rubber, has many uses in the automotive industry for tires, door and window profiles, seals such as O-rings and gaskets, hoses, belts, matting, and flooring. They offer a different range of physical and chemical properties, so can improve the reliability of a given product or application. Synthetic rubbers are superior to natural rubbers in two major respects, thermal stability and resistance to oils and related compounds. They are more resistant to oxidizing agents, such as oxygen and ozone which can reduce the life of products like tires.

Polybutadiene [butadiene rubber BR] is a synthetic rubber. Polybutadiene rubber is a polymer formed from the polymerization of the monomer 1,3-butadiene. Polybutadiene has a high resistance to wear and is used especially in the manufacture of tires, which consumes about 70% of the production. Another 25% is used as an additive to improve the toughness of plastics such as polystyrene and acrylonitrile butadiene styrene (ABS). Polybutadiene rubber accounted for about a quarter of total global consumption of synthetic rubbers in 2012. It is also used to manufacture golf balls, various elastic objects and to coat or encapsulate electronic assemblies, offering high electrical resistivity. Polybutadiene is typically crosslinked with sulphur, however, it has also been shown that it can be UV cured when bis-benzophenone additives are incorporated into the formulation.

β-Hydride elimination is a reaction in which an alkyl group bonded to a metal centre is converted into the corresponding metal-bonded hydride and an alkene. The alkyl must have hydrogens on the β-carbon. For instance butyl groups can undergo this reaction but methyl groups cannot. The metal complex must have an empty site cis to the alkyl group for this reaction to occur. Moreover, for facile cleavage of the C–H bond, a d electron pair is needed for donation into the σ* orbital of the C–H bond. Thus, d0 metals alkyls are generally more stable to β-hydride elimination than d2 and higher metal alkyls and may form isolable agostic complexes, even if an empty coordination site is available.
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More specialized polyolefins include polyisobutylene and polymethylpentene. They are all colorless or white oils or solids. Many copolymers are known, such as polybutene, which derives from a mixture of different butene isomers. The name of each polyolefin indicates the olefin from which it is prepared; for example, polyethylene is derived from ethylene, and polymethylpentene is derived from 4-methyl-1-pentene. Polyolefins are not olefins themselves because the double bond of each olefin monomer is opened in order to form the polymer. Monomers having more than one double bond such as butadiene and isoprene yield polymers that contain double bonds (polybutadiene and polyisoprene) and are usually not considered polyolefins. Polyolefins are the foundations of many chemical industries.
The Cossee–Arlman mechanism in polymer chemistry is the main pathway for the formation of C–C bonds in the polymerization of alkenes. The mechanism features an intermediate coordination complex that contains both the growing polymer chain and the monomer (alkene). These ligands combine within the coordination sphere of the metal to form a polymer chain that is elongated by two carbons.

Latex is an emulsion of polymer microparticles in water. Latexes are found in nature, but synthetic latexes are common as well.

The Charles Goodyear Medal is the highest honor conferred by the American Chemical Society, Rubber Division. Established in 1941, the award is named after Charles Goodyear, the discoverer of vulcanization, and consists of a gold medal, a framed certificate and prize money. The medal honors individuals for "outstanding invention, innovation, or development which has resulted in a significant change or contribution to the nature of the rubber industry". Awardees give a lecture at an ACS Rubber Division meeting, and publish a review of their work in the society's scientific journal Rubber Chemistry and Technology.
Samuel Emmett Horne Jr. was a research scientist at B. F. Goodrich noted for first synthesizing cis-1,4-polyisoprene, the main polymer contained in natural tree rubber, using Ziegler catalysis. Earlier attempts to produce synthetic rubber from isoprene had been unsuccessful, but in 1955, Horne prepared 98 percent cis-1,4-polyisoprene via the stereospecific polymerization of isoprene. The product of this reaction differs from natural rubber only slightly. It contains a small amount of cis-1,2-polyisoprene, but it is indistinguishable from natural rubber in its physical properties.
Synthetic biopolymers are human-made copies of biopolymers obtained by abiotic chemical routes. Synthetic biopolymer of different chemical nature have been obtained, including polysaccharides, glycoproteins, peptides and proteins, polyhydroxoalkanoates, polyisoprenes.
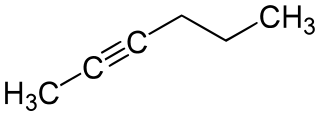
2-Hexyne is an organic compound that belongs to the alkyne group. Just like its isomers, it also has the chemical formula of C6H10.






![<span class="mw-page-title-main">Polyacetylene</span> Organic polymer made of the repeating unit [C2H2]](https://upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Cis-and-trans-polyacetylene-chains-symmetric-8-based-on-xtals-3D-bs-17.png/320px-Cis-and-trans-polyacetylene-chains-symmetric-8-based-on-xtals-3D-bs-17.png)






