
Polyurethane foam is a specialist material used for thermal insulation and other applications. It is a solid polymeric foam based on polyurethane chemistry.

Polyurethane foam is a specialist material used for thermal insulation and other applications. It is a solid polymeric foam based on polyurethane chemistry.
The so-called flexible polyurethane foam (FPF) is produced from the reaction of polyols and isocyanates, a process pioneered in 1937. [1] FPF allows for some compression and resilience that provides a cushioning effect. Because of this property, it is often used in furniture, bedding, automotive seating, athletic equipment, packaging, footwear and carpets. [1]
Rigid polyurethane foam has many desirable properties which has enabled increased use in various applications, some of which are quite demanding. [2] [3] These properties include low thermal conduction making it useful as an insulator. It also has low density compared to metals and other materials and also good dimensional stability. [4] A metal will expand on heating whereas rigid PU foam does not. They have excellent strength to weight ratios. [5] Like many applications, there has been a trend to make rigid PU foam from renewable raw materials in place of the usual polyols. [6] [7] [8]
They are used in vehicles, planes and buildings in structural applications. [9] They have also been used in fire-retardant applications. [10]
Polyurethane foam has been widely used to insulate fuel tanks on Space Shuttles. However, it requires a perfect application, as any air pocket, dirt or an uncovered tiny spot can knock it off due to extreme conditions of liftoff. [11] Those conditions include violent vibrations, air friction and abrupt changes in temperature and pressure. For a perfect application of the foam there have been two obstacles: limitations related to wearing protective suits and masks by workers and inability to test for cracks before launch, such testing is done only by naked eye. [11] The loss of foam caused the Space Shuttle Columbia disaster. According to the Columbia accident report, NASA officials found foam loss in over 80% of the 79 missions for which they have pictures. [11]
By 2009 researchers created a superior polyimide foam to insulate the reusable cryogenic propellant tanks of Space Shuttles. [12]

Polyurethane refers to a class of polymers composed of organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane is produced from a wide range of starting materials. This chemical variety produces polyurethanes with different chemical structures leading to many different applications. These include rigid and flexible foams, and coatings, adhesives, electrical potting compounds, and fibers such as spandex and polyurethane laminate (PUL). Foams are the largest application accounting for 67% of all polyurethane produced in 2016.

In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening ("curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and may be promoted by high pressure or mixing with a catalyst. Heat is not necessarily applied externally, and is often generated by the reaction of the resin with a curing agent. Curing results in chemical reactions that create extensive cross-linking between polymer chains to produce an infusible and insoluble polymer network.

Memory foam consists mainly of polyurethane with additional chemicals that increase its viscosity and density. It is often referred to as "viscoelastic" polyurethane foam, or low-resilience polyurethane foam (LRPu). The foam bubbles or ‘cells’ are open, effectively creating a matrix through which air can move. Higher-density memory foam softens in reaction to body heat, allowing it to mold to a warm body in a few minutes. Newer foams may recover their original shape more quickly.
In organic chemistry, a polyol is an organic compound containing multiple hydroxyl groups. The term "polyol" can have slightly different meanings depending on whether it is used in food science or polymer chemistry. Polyols containing two, three and four hydroxyl groups are diols, triols, and tetrols, respectively.

Foam rubber refers to rubber that has been manufactured with a foaming agent to create an air-filled matrix structure. Commercial foam rubbers are generally made of synthetic rubber, natural latex or polyurethane. Latex foam rubber, used in mattresses, is well known for its endurance. Polyurethane is a thermosetting polymer that comes from combination of Methyl di-isocyanate and polyethylene and some other chemical additives.

Polyisocyanurate, also referred to as PIR, polyiso, or ISO, is a thermoset plastic typically produced as a foam and used as rigid thermal insulation. The starting materials are similar to those used in polyurethane (PUR) except that the proportion of methylene diphenyl diisocyanate (MDI) is higher and a polyester-derived polyol is used in the reaction instead of a polyether polyol. The resulting chemical structure is significantly different, with the isocyanate groups on the MDI trimerising to form isocyanurate groups which the polyols link together, giving a complex polymeric structure.
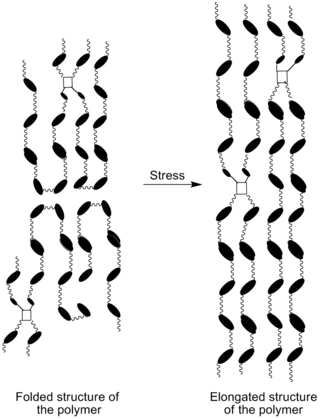
Strain crystallization is a phenomenon in which an initially amorphous solid material undergoes a phase transformation due to the application of strain. Strain crystallization occurs in natural rubber, as well as other elastomers and polymers. The phenomenon has important effects on strength and fatigue properties.

Melamine resin or melamine formaldehyde is a resin with melamine rings terminated with multiple hydroxyl groups derived from formaldehyde. This thermosetting plastic material is made from melamine and formaldehyde. In its butylated form, it is dissolved in n-butanol and xylene. It is then used to cross-link with alkyd, epoxy, acrylic, and polyester resins, used in surface coatings. There are many types, varying from very slow to very fast curing.
Reaction injection molding (RIM) is similar to injection molding except thermosetting polymers are used, which requires a curing reaction to occur within the mold.
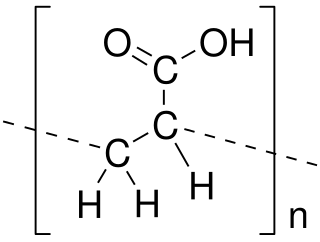
Poly(acrylic acid) (PAA; trade name Carbomer) is a polymer with the formula (CH2-CHCO2H)n. It is a derivative of acrylic acid (CH2=CHCO2H). In addition to the homopolymers, a variety of copolymers and crosslinked polymers, and partially deprotonated derivatives thereof are known and of commercial value. In a water solution at neutral pH, PAA is an anionic polymer, i.e., many of the side chains of PAA lose their protons and acquire a negative charge. Partially or wholly deprotonated PAAs are polyelectrolytes, with the ability to absorb and retain water and swell to many times their original volume. These properties – acid-base and water-attracting – are the bases of many applications.

Insulated pipes are widely used for district heating and hot water supply. They consist of a steel pipe called "service pipe", a thermal insulation layer and an outer casing. The insulation bonds the service pipe and the casing together. The main purpose of such pipes is to maintain the temperature of the fluid inside the service pipes. Insulated pipes are commonly used for transport of hot water from district heating plants to district heating networks and for distribution of hot water inside district heating networks.
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the Space Shuttle.
In polymer chemistry, the term prepolymer or pre-polymer, refers to a monomer or system of monomers that have been reacted to an intermediate-molecular mass state. This material is capable of further polymerization by reactive groups to a fully cured, high-molecular-mass state. As such, mixtures of reactive polymers with un-reacted monomers may also be referred to as pre-polymers. The term "pre-polymer" and "polymer precursor" may be interchanged.
Polyurethane dispersion, or PUD, is understood to be a polyurethane polymer resin dispersed in water, rather than a solvent, although some cosolvent maybe used. Its manufacture involves the synthesis of polyurethanes having carboxylic acid functionality or nonionic hydrophiles like PEG incorporated into, or pendant from, the polymer backbone. Two component polyurethane dispersions are also available.
Waterborne resins are sometimes called water-based resins. They are resins or polymeric resins that use water as the carrying medium as opposed to solvent or solvent-less. Resins are used in the production of coatings, adhesives, sealants, elastomers and composite materials. When the phrase waterborne resin is used, it usually describes all resins which have water as the main carrying solvent. The resin could be water-soluble, water reducible or water dispersed.
Hydrogenated MDI (H12MDI or 4,4′-diisocyanato dicyclohexylmethane) is an organic compound in the class known as isocyanates. More specifically, it is an aliphatic diisocyanate. It is a water white liquid at room temperature and is manufactured in relatively small quantities. It is also known as 4,4'-methylenedi(cyclohexyl isocyanate) or methylene bis(4-cyclohexylisocyanate) and has the formula CH2[(C6H10)NCO]2.
Biofoams are biological or biologically derived foams, making up lightweight and porous cellular solids. A relatively new term, its use in academia began in the 1980s in relation to the scum that formed on activated sludge plants.
Covalent adaptable networks (CANs) are a type of polymer material that closely resemble thermosetting polymers (thermosets). However, they are distinguished from thermosets by the incorporation of dynamic covalent chemistry into the polymer network. When a stimulus (for example heat, light, pH, ...) is applied to the material, these dynamic bonds become active and can be broken or exchanged with other pending functional groups, allowing the polymer network to change its topology. This introduces reshaping, (re)processing and recycling into thermoset-like materials.
Polyetheramines are a group of chemicals that are aliphatic organic species based on both ether and amine groups. They are produced by reacting either ethylene oxide or propylene oxide with polyols and then aminating them. There are a number of commercially available molecules with different CAS numbers and molecular weights. They often come with a prefix of M, D or T for monofunctional, difunctional and trifunctional respectively. D-230 would mean difunctional with a molecular weight of 230. A key use is for curing epoxy resins.