
The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the production of ammonia. It is named after its inventors, the German chemists: Fritz Haber and Carl Bosch, who developed it in the first decade of the 20th century. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using a metal catalyst under high temperatures and pressures. This reaction is slightly exothermic (i.e. it releases energy), meaning that the reaction is favoured at lower temperatures and higher pressures. It decreases entropy, complicating the process. Hydrogen is produced via steam reforming, followed by an iterative closed cycle to react hydrogen with nitrogen to produce ammonia.
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide, in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as a fuel. Historically, it has been used as a replacement for gasoline, when gasoline supply has been limited; for example, wood gas was used to power cars in Europe during WWII.

Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (CO2). This is achieved by reacting the feedstock material at high temperatures (typically >700 °C), without combustion, via controlling the amount of oxygen and/or steam present in the reaction. The resulting gas mixture is called syngas (from synthesis gas) or producer gas and is itself a fuel due to the flammability of the H2 and CO of which the gas is largely composed. Power can be derived from the subsequent combustion of the resultant gas, and is considered to be a source of renewable energy if the gasified compounds were obtained from biomass feedstock.

Energy development is the field of activities focused on obtaining sources of energy from natural resources. These activities include the production of renewable, nuclear, and fossil fuel derived sources of energy, and for the recovery and reuse of energy that would otherwise be wasted. Energy conservation and efficiency measures reduce the demand for energy development, and can have benefits to society with improvements to environmental issues.
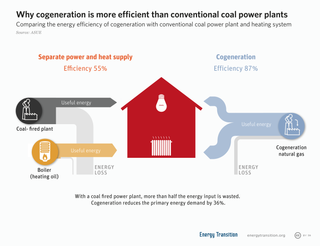
Cogeneration or combined heat and power (CHP) is the use of a heat engine or power station to generate electricity and useful heat at the same time.
The hydrogen economy uses hydrogen to decarbonize economic sectors which are hard to electrify, essentially, the "hard-to-abate" sectors such as cement, steel, long-haul transport, etc. In order to phase out fossil fuels and limit climate change, hydrogen can be created from water using renewable sources such as wind and solar, and its combustion only releases water vapor into the atmosphere.
Hydrogen fuel refers to hydrogen which is burned as fuel with oxygen. It can be a zero-carbon fuel, provided that it is created in a process that does not involve carbon. There are many types of hydrogen like white, green, blue, grey, black, or brown hydrogen owing to the various methods of processes by which they come. It can be used in fuel cells or internal combustion engines. Regarding hydrogen vehicles, hydrogen has begun to be used in commercial fuel cell vehicles such as passenger cars, and has been used in fuel cell buses for many years. It is also used as a fuel for spacecraft propulsion and is being proposed for hydrogen-powered aircraft. The fuel technology has seen awakened interest from automakers who claim it is comparatively cheap and safer to incorporate into the modern vehicle architecture over recent challenges faced by electric vehicle makers.

High-temperature electrolysis is a technology for producing hydrogen from water at high temperatures.
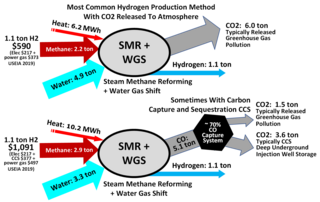
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is hydrogen production. The reaction is represented by this equilibrium:

The Sabatier reaction or Sabatier process produces methane and water from a reaction of hydrogen with carbon dioxide at elevated temperatures and pressures in the presence of a nickel catalyst. It was discovered by the French chemists Paul Sabatier and Jean-Baptiste Senderens in 1897. Optionally, ruthenium on alumina makes a more efficient catalyst. It is described by the following exothermic reaction:

Water splitting is the chemical reaction in which water is broken down into oxygen and hydrogen:

The sulfur–iodine cycle is a three-step thermochemical cycle used to produce hydrogen.
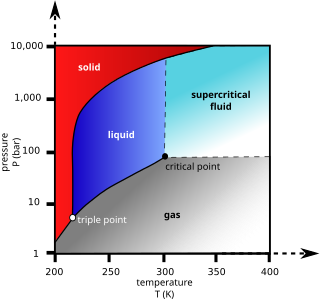
Supercritical carbon dioxide is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure.
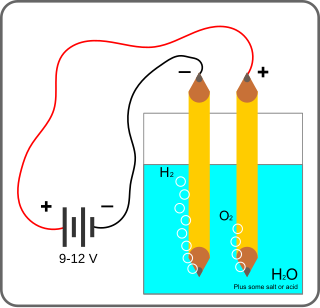
Electrolysis of water is using electricity to split water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, or remixed with the oxygen to create oxyhydrogen gas, for use in welding and other applications.

Electric heating is a process in which electrical energy is converted directly to heat energy. Common applications include space heating, cooking, water heating and industrial processes. An electric heater is an electrical device that converts an electric current into heat. The heating element inside every electric heater is an electrical resistor, and works on the principle of Joule heating: an electric current passing through a resistor will convert that electrical energy into heat energy. Most modern electric heating devices use nichrome wire as the active element; the heating element, depicted on the right, uses nichrome wire supported by ceramic insulators.

The steam-electric power station is a power station in which the electric generator is steam driven. Water is heated, turns into steam and spins a steam turbine which drives an electrical generator. After it passes through the turbine, the steam is condensed in a condenser. The greatest variation in the design of steam-electric power plants is due to the different fuel sources.
Hydrogen production is the family of industrial methods for generating hydrogen gas. As of 2020, the majority of hydrogen (~95%) is produced from fossil fuels by steam reforming of natural gas and other light hydrocarbons, partial oxidation of heavier hydrocarbons, and coal gasification. Other methods of hydrogen production include biomass gasification, methane pyrolysis, and electrolysis of water. Methane pyrolysis and water electrolysis can use any source of electricity including solar power.
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.
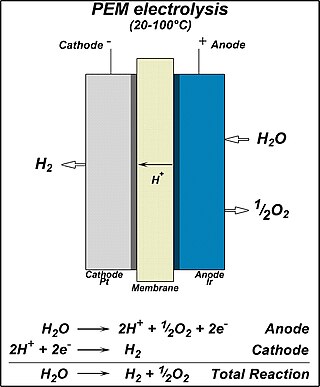
Proton exchange membrane(PEM) electrolysis is the electrolysis of water in a cell equipped with a solid polymer electrolyte (SPE) that is responsible for the conduction of protons, separation of product gases, and electrical insulation of the electrodes. The PEM electrolyzer was introduced to overcome the issues of partial load, low current density, and low pressure operation currently plaguing the alkaline electrolyzer. It involves a proton-exchange membrane.
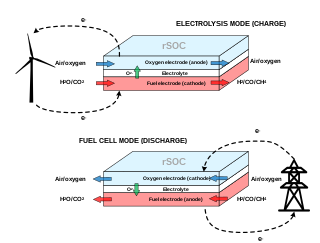
A reversible solid oxide cell (rSOC) is a solid-state electrochemical device that is operated alternatively as a solid oxide fuel cell (SOFC) and a solid oxide electrolysis cell (SOEC). Similarly to SOFCs, rSOCs are made of a dense electrolyte sandwiched between two porous electrodes. Their operating temperature ranges from 600°C to 900°C, hence they benefit from enhanced kinetics of the reactions and increased efficiency with respect to low-temperature electrochemical technologies.














