Related Research Articles

A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding.

In chemistry, a hydrogen bond is primarily an electrostatic force of attraction between a hydrogen (H) atom which is covalently bonded to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted Dn−H···Ac, where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the period 2 elements nitrogen (N), oxygen (O), and fluorine (F).
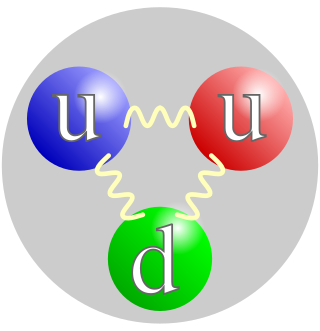
A proton is a stable subatomic particle, symbol
p
, H+, or 1H+ with a positive electric charge of +1 e (elementary charge). Its mass is slightly less than the mass of a neutron and 1,836 times the mass of an electron (the proton-to-electron mass ratio). Protons and neutrons, each with masses of approximately one atomic mass unit, are jointly referred to as "nucleons" (particles present in atomic nuclei).

A scanning tunneling microscope (STM) is a type of microscope used for imaging surfaces at the atomic level. Its development in 1981 earned its inventors, Gerd Binnig and Heinrich Rohrer, then at IBM Zürich, the Nobel Prize in Physics in 1986. STM senses the surface by using an extremely sharp conducting tip that can distinguish features smaller than 0.1 nm with a 0.01 nm (10 pm) depth resolution. This means that individual atoms can routinely be imaged and manipulated. Most scanning tunneling microscopes are built for use in ultra-high vacuum at temperatures approaching absolute zero, but variants exist for studies in air, water and other environments, and for temperatures over 1000 °C.
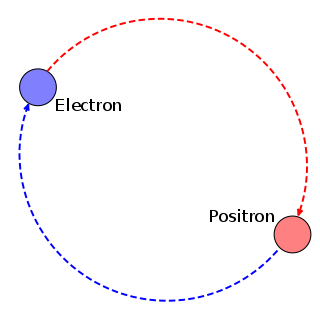
Positronium (Ps) is a system consisting of an electron and its anti-particle, a positron, bound together into an exotic atom, specifically an onium. Unlike hydrogen, the system has no protons. The system is unstable: the two particles annihilate each other to predominantly produce two or three gamma-rays, depending on the relative spin states. The energy levels of the two particles are similar to that of the hydrogen atom. However, because of the reduced mass, the frequencies of the spectral lines are less than half of those for the corresponding hydrogen lines.
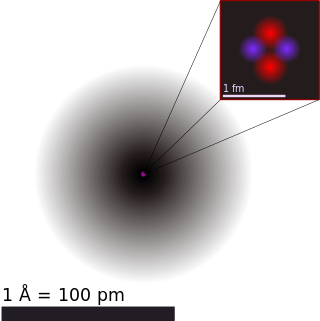
The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius. Four widely used definitions of atomic radius are: Van der Waals radius, ionic radius, metallic radius and covalent radius. Typically, because of the difficulty to isolate atoms in order to measure their radii separately, atomic radius is measured in a chemically bonded state; however theoretical calculations are simpler when considering atoms in isolation. The dependencies on environment, probe, and state lead to a multiplicity of definitions.

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.
In physics, quantum tunnelling, barrier penetration, or simply tunnelling is a quantum mechanical phenomenon in which an object such as an electron or atom passes through a potential energy barrier that, according to classical mechanics, should not be passable due to the object not having sufficient energy to pass or surmount the barrier.
An electron bubble is the empty space created around a free electron in a cryogenic gas or liquid, such as neon or helium. They are typically very small, about 2 nm in diameter at atmospheric pressure.

The hydrogen anion, H−, is a negative ion of hydrogen, that is, a hydrogen atom that has captured an extra electron. The hydrogen anion is an important constituent of the atmosphere of stars, such as the Sun. In chemistry, this ion is called hydride. The ion has two electrons bound by the electromagnetic force to a nucleus containing one proton.

Bond order potential is a class of empirical (analytical) interatomic potentials which is used in molecular dynamics and molecular statics simulations. Examples include the Tersoff potential, the EDIP potential, the Brenner potential, the Finnis–Sinclair potentials, ReaxFF, and the second-moment tight-binding potentials. They have the advantage over conventional molecular mechanics force fields in that they can, with the same parameters, describe several different bonding states of an atom, and thus to some extent may be able to describe chemical reactions correctly. The potentials were developed partly independently of each other, but share the common idea that the strength of a chemical bond depends on the bonding environment, including the number of bonds and possibly also angles and bond lengths. It is based on the Linus Pauling bond order concept and can be written in the form

The dihydrogen cation or hydrogen molecular ion is a cation with formula H+
2. It consists of two hydrogen nuclei (protons), each sharing a single electron. It is the simplest molecular ion.
The timeline of quantum mechanics is a list of key events in the history of quantum mechanics, quantum field theories and quantum chemistry.
In chemistry, ice rules are basic principles that govern arrangement of atoms in water ice. They are also known as Bernal–Fowler rules, after British physicists John Desmond Bernal and Ralph H. Fowler who first described them in 1933.
High-precision experiments could reveal small previously unseen differences between the behavior of matter and antimatter. This prospect is appealing to physicists because it may show that nature is not Lorentz symmetric.
The rms charge radius is a measure of the size of an atomic nucleus, particularly the proton distribution. The proton radius is about one femtometre = 10−15 metre. It can be measured by the scattering of electrons by the nucleus. Relative changes in the mean squared nuclear charge distribution can be precisely measured with atomic spectroscopy.
Bond softening is an effect of reducing the strength of a chemical bond by strong laser fields. To make this effect significant, the strength of the electric field in the laser light has to be comparable with the electric field the bonding electron "feels" from the nuclei of the molecule. Such fields are typically in the range of 1–10 V/Å, which corresponds to laser intensities 1013–1015 W/cm2. Nowadays, these intensities are routinely achievable from table-top Ti:Sapphire lasers.

In physics, the hydrodynamic quantum analogs refer to experimentally-observed phenomena involving bouncing fluid droplets over a vibrating fluid bath that behave analogously to several quantum-mechanical systems. The experimental evidence has been disputed.
Quantum microscopy allows microscopic properties of matter and quantum particles to be measured and imaged. Various types of microscopy use quantum principles. The first microscope to do so was the scanning tunneling microscope, which paved the way for development of the photoionization microscope and the quantum entanglement microscope.
The quantum tunneling of water occurs when water molecules in nanochannels exhibit quantum tunneling behavior that smears out the positions of the hydrogen atoms into a pair of correlated rings. In that state, the water molecules become delocalized around a ring and assume an unusual double top-like shape. At low temperatures, the phenomenon showcases the quantum motion of water through the separating potential walls, which is forbidden in classical mechanics, but allowed in quantum mechanics.
References
- ↑ Löwdin, P. O. (1963). "Proton Tunneling in DNA and its Biological Implications". Rev. Mod. Phys. 35 (3): 724–732. Bibcode:1963RvMP...35..724L. doi:10.1103/RevModPhys.35.724.
- ↑ Castro Neto, A. H.; Pujol, P.; Fradkin, E. (2006). "Ice: A strongly correlated proton system". Phys. Rev. B. 74 (2): 024302. arXiv: cond-mat/0511092 . Bibcode:2006PhRvB..74b4302C. doi:10.1103/PhysRevB.74.024302. S2CID 102581583.
- ↑ Drechsel-Grau, C.; Marx, D. (2014). "Quantum Simulation of Collective Proton Tunneling in Hexagonal Ice Crystals". Phys. Rev. Lett. 112 (14): 148302. Bibcode:2014PhRvL.112n8302D. doi:10.1103/PhysRevLett.112.148302. PMID 24766024.
- ↑ Yen, F.; Gao, T. (2015). "Dielectric Anomaly in Ice near 20 K: Evidence of Macroscopic Quantum Phenomena". J. Phys. Chem. Lett. 6 (14): 2822–2825. arXiv: 1508.00215 . doi:10.1021/acs.jpclett.5b00797. PMID 26266868. S2CID 38375110.
- ↑ Meng, X. Z.; et al. (2015). "Direct visualization of concerted proton tunnelling in a water nanocluster". Nat. Phys. 11 (3): 235–239. Bibcode:2015NatPh..11..235M. doi: 10.1038/NPHYS3225 .