In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid, a.k.a. phosphoric acid H3PO4.

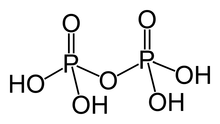
In chemistry, pyrophosphates are phosphorus oxyanions that contain two phosphorus atoms in a P−O−P linkage. A number of pyrophosphate salts exist, such as disodium pyrophosphate and tetrasodium pyrophosphate, among others. Often pyrophosphates are called diphosphates. The parent pyrophosphates are derived from partial or complete neutralization of pyrophosphoric acid. The pyrophosphate bond is also sometimes referred to as a phosphoanhydride bond, a naming convention which emphasizes the loss of water that occurs when two phosphates form a new P−O−P bond, and which mirrors the nomenclature for anhydrides of carboxylic acids. Pyrophosphates are found in ATP and other nucleotide triphosphates, which are important in biochemistry. The term pyrophosphate is also the name of esters formed by the condensation of a phosphorylated biological compound with inorganic phosphate, as for dimethylallyl pyrophosphate. This bond is also referred to as a high-energy phosphate bond.

Phosphoric acid is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula H3PO4. It is commonly encountered as an 85% aqueous solution, which is a colourless, odourless, and non-volatile syrupy liquid. It is a major industrial chemical, being a component of many fertilizers.
A polyphosphate is a salt or ester of polymeric oxyanions formed from tetrahedral PO4 (phosphate) structural units linked together by sharing oxygen atoms. Polyphosphates can adopt linear or a cyclic (also called, ring) structures. In biology, the polyphosphate esters ADP and ATP are involved in energy storage. A variety of polyphosphates find application in mineral sequestration in municipal waters, generally being present at 1 to 5 ppm. GTP, CTP, and UTP are also nucleotides important in the protein synthesis, lipid synthesis, and carbohydrate metabolism, respectively. Polyphosphates are also used as food additives, marked E452.

Trisodium phosphate (TSP) is an inorganic compound with the chemical formula Na3PO4. It is a white, granular or crystalline solid, highly soluble in water, producing an alkaline solution. TSP is used as a cleaning agent, builder, lubricant, food additive, stain remover, and degreaser.
An oxyanion, or oxoanion, is an ion with the generic formula A
xOz−
y. Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determined by the octet rule. The corresponding oxyacid of an oxyanion is the compound H
zA
xO
y. The structures of condensed oxyanions can be rationalized in terms of AOn polyhedral units with sharing of corners or edges between polyhedra. The oxyanions adenosine monophosphate (AMP), adenosine diphosphate (ADP) and adenosine triphosphate (ATP) are important in biology.

Sodium hexametaphosphate (SHMP) is a salt of composition Na6[(PO3)6]. Sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: NaPO3), of which the hexamer is one, and is usually the compound referred to by this name. Such a mixture is more correctly termed sodium polymetaphosphate. They are white solids that dissolve in water.

A sodium phosphate is a generic variety of salts of sodium and phosphate. Phosphate also forms families or condensed anions including di-, tri-, tetra-, and polyphosphates. Most of these salts are known in both anhydrous (water-free) and hydrated forms. The hydrates are more common than the anhydrous forms.

Tetrasodium pyrophosphate, also called sodium pyrophosphate, tetrasodium phosphate or TSPP, is an inorganic compound with the formula Na4P2O7. As a salt, it is a white, water-soluble solid. It is composed of pyrophosphate anion and sodium ions. Toxicity is approximately twice that of table salt when ingested orally. Also known is the decahydrate Na4P2O7 · 10(H2O).

Phosphorous acid is the compound described by the formula H3PO3. This acid is diprotic, not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO3H2, are called phosphonic acids.

In chemistry, a phosphoric acid, in the general sense, is a phosphorus oxoacid in which each phosphorus (P) atom is in the oxidation state +5, and is bonded to four oxygen (O) atoms, one of them through a double bond, arranged as the corners of a tetrahedron. Two or more of these PO4 tetrahedra may be connected by shared single-bonded oxygens, forming linear or branched chains, cycles, or more complex structures. The single-bonded oxygen atoms that are not shared are completed with acidic hydrogen atoms. The general formula of a phosphoric acid is Hn+2−2xPnO3n+1−x, where n is the number of phosphorus atoms and x is the number of fundamental cycles in the molecule's structure, between 0 and n + 2/2.

Phosphorus pentoxide is a chemical compound with molecular formula P4O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrating agent.

Tricalcium phosphate (sometimes abbreviated TCP), more commonly known as Calcium phosphate, is a calcium salt of phosphoric acid with the chemical formula Ca3(PO4)2. It is also known as tribasic calcium phosphate and bone phosphate of lime (BPL). It is a white solid of low solubility. Most commercial samples of "tricalcium phosphate" are in fact hydroxyapatite.

Monocalcium phosphate is an inorganic compound with the chemical formula Ca(H2PO4)2 ("AMCP" or "CMP-A" for anhydrous monocalcium phosphate). It is commonly found as the monohydrate ("MCP" or "MCP-M"), Ca(H2PO4)2·H2O. Both salts are colourless solids. They are used mainly as superphosphate fertilizers and are also popular leavening agents.
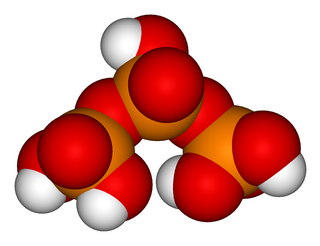
Triphosphoric acid (also tripolyphosphoric acid), with formula H5P3O10, is a condensed form of phosphoric acid. In the family of phosphoric acids, it is the next polyphosphoric acid after pyrophosphoric acid, H4P2O7, also called diphosphoric acid.
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bonded to oxygen that can dissociate to produce the H+ cation and the anion of the acid.
Dicalcium phosphate is the calcium phosphate with the formula CaHPO4 and its dihydrate. The "di" prefix in the common name arises because the formation of the HPO42– anion involves the removal of two protons from phosphoric acid, H3PO4. It is also known as dibasic calcium phosphate or calcium monohydrogen phosphate. Dicalcium phosphate is used as a food additive, it is found in some toothpastes as a polishing agent and is a biomaterial.

Disodium phosphate (DSP), or disodium hydrogen phosphate, or sodium phosphate dibasic, is an inorganic compound with the chemical formula Na2HPO4. It is one of several sodium phosphates. The salt is known in anhydrous form as well as hydrates Na2HPO4·nH2O, where n is 2, 7, 8, and 12. All are water-soluble white powders. The anhydrous salt is hygroscopic.

Monosodium phosphate (MSP), also known as monobasic sodium phosphate and sodium dihydrogen phosphate, is an inorganic compound with the chemical formula NaH2PO4. It is a sodium salt of phosphoric acid. It consists of sodium cations (Na+) and dihydrogen phosphate anions (H2PO−4). One of many sodium phosphates, it is a common industrial chemical. The salt exists in an anhydrous form, as well as monohydrate and dihydrate (NaH2PO4·H2O and NaH2PO4·2H2O respectively).
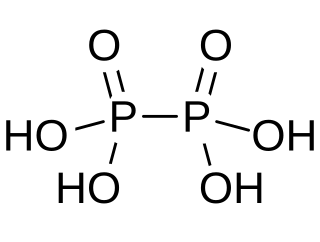
Hypophosphoric acid is a mineral acid with the formula H4P2O6, with phosphorus in a formal oxidation state of +4. In the solid state it is present as the dihydrate, H4P2O6·2H2O. In hypophosphoric acid the phosphorus atoms are identical and joined directly with a P−P bond. Isohypophosphoric acid is a structural isomer of hypophosphoric acid in which one phosphorus has a hydrogen directedly bonded to it and that phosphorus atom is linked to the other one by an oxygen bridge to give a phosphorous acid/phosphoric acid mixed anhydride. The two phosphorus atoms are in the +3 and +5 oxidation states, respectively.