Atmospheric variation
In the early years of using the technique, it was understood that it depended on the atmospheric 14
C/12
C ratio having remained the same over the preceding few thousand years. To verify the accuracy of the method, several artefacts that were datable by other techniques were tested; the results of the testing were in reasonable agreement with the true ages of the objects. However, in 1958, Hessel de Vries was able to demonstrate that the 14
C/12
C ratio had changed over time by testing wood samples of known ages and showing there was a significant deviation from the expected ratio. This discrepancy, often called the de Vries effect, was resolved by the study of tree rings. [1] [2] Comparison of overlapping series of tree rings allowed the construction of a continuous sequence of tree-ring data that spanned 8,000 years. [1] (Since that time the tree-ring data series has been extended to 13,900 years.) [3] Carbon-dating the wood from the tree rings themselves provided the check needed on the atmospheric 14
C/12
C ratio: with a sample of known date, and a measurement of the value of N (the number of atoms of 14
C remaining in the sample), the carbon-dating equation allows the calculation of N0 – the number of atoms of 14
C in the sample at the time the tree ring was formed – and hence the 14
C/12
C ratio in the atmosphere at that time. [1] Armed with the results of carbon-dating the tree rings, it became possible to construct calibration curves designed to correct the errors caused by the variation over time in the 14
C/12
C ratio. [4] These curves are described in more detail below.
There are three main reasons for these variations in the historical 14
C/12
C ratio: fluctuations in the rate at which 14
C is created, changes caused by glaciation, and changes caused by human activity. [1]
Variations in 14
C production
Two different trends can be seen in the tree ring series. First, there is a long-term oscillation with a period of about 9,000 years, which causes radiocarbon dates to be older than true dates for the last 2,000 years and too young before that. The known fluctuations in the strength of the Earth's magnetic field match up quite well with this oscillation: cosmic rays are deflected by magnetic fields, so when there is a weaker magnetic field, more 14
C is produced, leading to a younger apparent age for samples from those periods. Conversely, a stronger magnetic field leads to lower 14
C production and an older apparent age. A secondary oscillation is thought to be caused by variations in sunspot activity, which has two separate periods: a longer-term, 200-year oscillation, and a shorter 11-year cycle. Sunspots cause changes in the solar system's magnetic field and corresponding changes to the cosmic ray flux, and hence to the production of 14
C. [1]
There are two kinds of geophysical event which can affect 14
C production: geomagnetic reversals and polarity excursions. In a geomagnetic reversal, the Earth's geomagnetic field weakens and stays weak for thousands of years during the transition to the opposite magnetic polarity and then regains strength as the reversal completes. A polarity excursion, which can be either global or local, is a shorter-lived version of a geomagnetic reversal. A local excursion would not significantly affect 14C production. During either a geomagnetic reversal or a global polarity excursion, 14
C production increases during the period when the geomagnetic field is weak. It is fairly certain, though, that in the last 50,000 years there have been no geomagnetic reversals or global polarity excursions. [5]
Since the earth's magnetic field varies with latitude, the rate of 14
C production changes with latitude, too, but atmospheric mixing is rapid enough that these variations amount to less than 0.5% of the global concentration. [1] This is close to the limit of detectability in most years, [6] but the effect can be seen clearly in tree rings from years such as 1963, when 14
C from nuclear testing rose sharply through the year. [7] The latitudinal variation in 14
C was much larger than normal that year, and tree rings from different latitudes show corresponding variations in their 14
C content. [7]
14
C can also be produced at ground level, primarily by cosmic rays that penetrate the atmosphere as far as the earth's surface, but also by spontaneous fission of naturally occurring uranium. These sources of neutrons only produce 14
C at a rate of 1 x 10−4 atoms per gram per second, which is not enough to have a significant effect on dating. [7] [8] At higher altitudes, the neutron flux can be substantially higher, [9] [note 1] and in addition, trees at higher altitude are more likely to be struck by lightning, which produces neutrons. However, experiments in which wood samples have been irradiated with neutrons indicate that the effect on 14
C content is minor, though for very old trees (such as some bristlecone pines) that grow at altitude some effect can be seen. [9]
Effect of climatic cycles
Because the solubility of CO
2 in water increases with lower temperatures, glacial periods would have led to the faster absorption of atmospheric CO
2 by the oceans. In addition, any carbon stored in the glaciers would be depleted in 14
C over the life of the glacier; when the glacier melted as the climate warmed, the depleted carbon would be released, reducing the global 14
C/12
C ratio. The changes in climate would also cause changes in the biosphere, with warmer periods leading to more plant and animal life. The effect of these factors on radiocarbon dating is not known. [1]
Effects of human activity

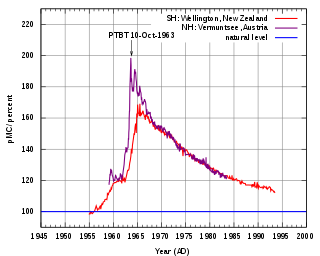
C for the northern and southern hemispheres, showing percentage excess above pre-bomb levels. The Partial Test Ban Treaty went into effect on 10 October 1963.
Coal and oil began to be burned in large quantities during the 1800s. Both coal and oil are sufficiently old that they contain little detectable 14
C and, as a result, the CO
2 released substantially diluted the atmospheric 14
C/12
C ratio. Dating an object from the early 20th century hence gives an apparent date older than the true date. For the same reason, 14
C concentrations in the neighbourhood of large cities are lower than the atmospheric average. This fossil fuel effect (also known as the Suess effect, after Hans Suess, who first reported it in 1955) would only amount to a reduction of 0.2% in 14
C activity if the additional carbon from fossil fuels were distributed throughout the carbon exchange reservoir, but because of the long delay in mixing with the deep ocean, the actual effect is a 3% reduction. [1] [11]
A much larger effect comes from above-ground nuclear testing, which released large numbers of neutrons and created 14
C. From about 1950 until 1963, when atmospheric nuclear testing was banned, it is estimated that several tonnes of 14
C were created. If all this extra 14
C had immediately been spread across the entire carbon exchange reservoir, it would have led to an increase in the 14
C/12
C ratio of only a few per cent, but the immediate effect was to almost double the amount of 14
C in the atmosphere, with the peak level occurring in about 1965. The level has since dropped, as the "bomb carbon" (as it is sometimes called) percolates into the rest of the reservoir. [1] [11] [12]