
Macrophages are a type of white blood cell of the innate immune system that engulf and digest pathogens, such as cancer cells, microbes, cellular debris, and foreign substances, which do not have proteins that are specific to healthy body cells on their surface. This process is called phagocytosis, which acts to defend the host against infection and injury.

Monocytes are a type of leukocyte or white blood cell. They are the largest type of leukocyte in blood and can differentiate into macrophages and monocyte-derived dendritic cells. As a part of the vertebrate innate immune system monocytes also influence adaptive immune responses and exert tissue repair functions. There are at least three subclasses of monocytes in human blood based on their phenotypic receptors.
In immunology, the mononuclear phagocyte system or mononuclear phagocytic system (MPS) also known as the reticuloendothelial system or macrophage system is a part of the immune system that consists of the phagocytic cells located in reticular connective tissue. The cells are primarily monocytes and macrophages, and they accumulate in lymph nodes and the spleen. The Kupffer cells of the liver and tissue histiocytes are also part of the MPS. The mononuclear phagocyte system and the monocyte macrophage system refer to two different entities, often mistakenly understood as one.

Kupffer cells, also known as stellate macrophages and Kupffer–Browicz cells, are specialized cells localized in the liver within the lumen of the liver sinusoids and are adhesive to their endothelial cells which make up the blood vessel walls. Kupffer cells comprise the largest population of tissue-resident macrophages in the body. Gut bacteria, bacterial endotoxins, and microbial debris transported to the liver from the gastrointestinal tract via the portal vein will first come in contact with Kupffer cells, the first immune cells in the liver. It is because of this that any change to Kupffer cell functions can be connected to various liver diseases such as alcoholic liver disease, viral hepatitis, intrahepatic cholestasis, steatohepatitis, activation or rejection of the liver during liver transplantation and liver fibrosis. They form part of the mononuclear phagocyte system.

Microglia are a type of neuroglia located throughout the brain and spinal cord. Microglia account for about 10-15% of cells found within the brain. As the resident macrophage cells, they act as the first and main form of active immune defense in the central nervous system (CNS). Microglia originate in the yolk sac under a tightly regulated molecular process. These cells are distributed in large non-overlapping regions throughout the CNS. Microglia are key cells in overall brain maintenance—they are constantly scavenging the CNS for plaques, damaged or unnecessary neurons and synapses, and infectious agents. Since these processes must be efficient to prevent potentially fatal damage, microglia are extremely sensitive to even small pathological changes in the CNS. This sensitivity is achieved in part by the presence of unique potassium channels that respond to even small changes in extracellular potassium. Recent evidence shows that microglia are also key players in the sustainment of normal brain functions under healthy conditions. Microglia also constantly monitor neuronal functions through direct somatic contacts and exert neuroprotective effects when needed.
A histiocyte is a vertebrate cell that is part of the mononuclear phagocyte system. The mononuclear phagocytic system is part of the organism's immune system. The histiocyte is a tissue macrophage or a dendritic cell. Part of their job is to clear out neutrophils once they've reached the end of their lifespan.
Scavenger receptors are a large and diverse superfamily of cell surface receptors. Its properties were first recorded in 1970 by Drs. Brown and Goldstein, with the defining property being the ability to bind and remove modified low density lipoproteins (LDL). Today scavenger receptors are known to be involved in a wide range of processes, such as: homeostasis, apoptosis, inflammatory diseases and pathogen clearance. Scavenger receptors are mainly found on myeloid cells and other cells that bind to numerous ligands, primarily endogenous and modified host-molecules together with pathogen-associated molecular patterns(PAMPs), and remove them. The Kupffer cells in the liver are particularly rich in scavenger receptors, includes SR-A I, SR-A II, and MARCO.

Hemosiderin or haemosiderin is an iron-storage complex that is composed of partially digested ferritin and lysosomes. The breakdown of heme gives rise to biliverdin and iron. The body then traps the released iron and stores it as hemosiderin in tissues. Hemosiderin is also generated from the abnormal metabolic pathway of ferritin.
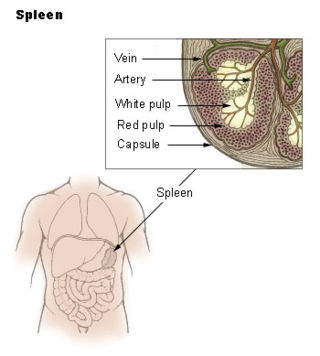
The red pulp of the spleen is composed of connective tissue known also as the cords of Billroth and many splenic sinusoids that are engorged with blood, giving it a red color. Its primary function is to filter the blood of antigens, microorganisms, and defective or worn-out red blood cells.

A liver sinusoid is a type of capillary known as a sinusoidal capillary, discontinuous capillary or sinusoid, that is similar to a fenestrated capillary, having discontinuous endothelium that serves as a location for mixing of the oxygen-rich blood from the hepatic artery and the nutrient-rich blood from the portal vein.
The mannose receptor is a C-type lectin primarily present on the surface of macrophages, immature dendritic cells and liver sinusoidal endothelial cells, but is also expressed on the surface of skin cells such as human dermal fibroblasts and keratinocytes. It is the first member of a family of endocytic receptors that includes Endo180 (CD280), M-type PLA2R, and DEC-205 (CD205).

CD68 is a protein highly expressed by cells in the monocyte lineage, by circulating macrophages, and by tissue macrophages.

According to a common point of view epithelioid cells are derivatives of activated macrophages resembling epithelial cells.

CD47 also known as integrin associated protein (IAP) is a transmembrane protein that in humans is encoded by the CD47 gene. CD47 belongs to the immunoglobulin superfamily and partners with membrane integrins and also binds the ligands thrombospondin-1 (TSP-1) and signal-regulatory protein alpha (SIRPα). CD-47 acts as a don't eat me signal to macrophages of the immune system which has made it a potential therapeutic target in some cancers, and more recently, for the treatment of pulmonary fibrosis.

Stabilin-1 is a protein that in humans is encoded by the STAB1 gene.
Liver cytology is the branch of cytology that studies the liver cells and its functions. The liver is a vital organ, in charge of almost all the body’s metabolism. Main liver cells are hepatocytes, Kupffer cells, and hepatic stellate cells; each one with a specific function.
Clark Lawrence Anderson is an internist and immunologist. He is professor emeritus in the Division of Immunology and Rheumatology, Department of Internal Medicine, Ohio State University (OSU), Columbus, Ohio, United States.
Liver sinusoidal endothelial cells (LSECs) form the lining of the smallest blood vessels in the liver, also called the hepatic sinusoids. LSECs are highly specialized endothelial cells with characteristic morphology and function. They constitute an important part of the reticuloendothelial system (RES).
The term scavenger endothelial cell (SEC) was initially coined to describe a specialized sub-group of endothelial cells in vertebrates that express a remarkably high blood clearance activity. The term SEC has now been adopted by several scientists.
Endothelial cell tropism or endotheliotropism is a type of tissue tropism or host tropism that characterizes an pathogen's ability to recognize and infect an endothelial cell. Pathogens, such as viruses, can target a specific tissue type or multiple tissue types. Like other cells, the endothelial cell possesses several features that supports a productive viral infection a cell including, cell surface receptors, immune responses, and other virulence factors. Endothelial cells are found in various tissue types such as in the capillaries, veins, and arteries in the human body. As endothelial cells line these blood vessels and critical networks that extend access to various human organ systems, the virus entry into these cells can be detrimental to virus spread across the host system and affect clinical course of disease. Understanding the mechanisms of how viruses attach, enter, and control endothelial functions and host responses inform infectious disease understanding and medical countermeasures.










