
A mycorrhiza is a symbiotic association between a fungus and a plant. The term mycorrhiza refers to the role of the fungus in the plant's rhizosphere, its root system. Mycorrhizae play important roles in plant nutrition, soil biology, and soil chemistry.

A truffle is the fruiting body of a subterranean ascomycete fungus, one of the species of the genus Tuber. More than one hundred other genera of fungi are classified as truffles including Geopora, Peziza, Choiromyces, and Leucangium. These genera belong to the class Pezizomycetes and the Pezizales order. Several truffle-like basidiomycetes are excluded from Pezizales, including Rhizopogon and Glomus. Truffles are ectomycorrhizal fungi, so they are found in close association with tree roots. Spore dispersal is accomplished through fungivores, animals that eat fungi. These fungi have ecological roles in nutrient cycling and drought tolerance.

The Russulaceae are a diverse family of fungi in the order Russulales, with roughly 1,900 known species and a worldwide distribution. They comprise the brittlegills and the milk-caps, well-known mushroom-forming fungi that include some edible species. These gilled mushrooms are characterised by the brittle flesh of their fruitbodies.

Pterospora, commonly known as pinedrops, woodland pinedrops, Albany beechdrops, or giant bird's nest, is a North American genus in the subfamily Monotropoideae of the heath family, and includes only the species Pterospora andromedea. It grows as a mycoheterotroph in coniferous or mixed forests. It is widespread across much of Canada as well as the western and northeastern United States to Mexico. Along with Monotropa it is one of the more frequently encountered genera of the Monotropoideae.

Lactarius is a genus of mushroom-producing, ectomycorrhizal fungi, containing several edible species. The species of the genus, commonly known as milk-caps, are characterized by the milky fluid ("latex") they exude when cut or damaged. Like the closely related genus Russula, their flesh has a distinctive brittle consistency. It is a large genus with over 500 known species, mainly distributed in the Northern hemisphere. Recently, the genus Lactifluus has been separated from Lactarius based on molecular phylogenetic evidence.

Suillus luteus is a bolete fungus, and the type species of the genus Suillus. A common fungus native all across Eurasia from Ireland to Korea, it has been introduced widely elsewhere, including North and South America, southern Africa, Australia and New Zealand. Commonly referred to as slippery jack or sticky bun in English-speaking countries, its names refer to the brown cap, which is characteristically slimy in wet conditions. The fungus, initially described as Boletus luteus by Carl Linnaeus in 1753, is now classified in a different fungus family as well as genus. Suillus luteus is edible, though not as highly regarded as other bolete mushrooms. It is commonly prepared and eaten in soups, stews or fried dishes. The slime coating, however, may cause indigestion if not removed before eating. It is often sold as a dried mushroom.

Laccaria bicolor is a small tan-colored mushroom with lilac gills. It is edible but not choice, and grows in mixed birch and pine woods. It is found in the temperate zones of the globe, in late summer and autumn. L. bicolor is an ectomycorrhizal fungus used as a soil inoculant in agriculture and horticulture.

Suillus bovinus, also known as the Jersey cow mushroom or bovine bolete, is a pored mushroom of the genus Suillus in the family Suillaceae. A common fungus native to Europe and Asia, it has been introduced to North America and Australia. It was initially described as Boletus bovinus by Carl Linnaeus in 1753, and given its current binomial name by Henri François Anne de Roussel in 1806. It is an edible mushroom, though not highly regarded.

The sporocarp of fungi is a multicellular structure on which spore-producing structures, such as basidia or asci, are borne. The fruitbody is part of the sexual phase of a fungal life cycle, while the rest of the life cycle is characterized by vegetative mycelial growth and asexual spore production.

Suillus brevipes is a species of fungus in the family Suillaceae. First described by American mycologists in the late 19th century, it is commonly known as the stubby-stalk or the short-stemmed slippery Jack. The fruit bodies (mushrooms) produced by the fungus are characterized by a chocolate to reddish-brown cap covered with a sticky layer of slime, and a short whitish stipe that has neither a partial veil nor prominent, colored glandular dots. The cap can reach a diameter of about 10 cm, while the stipe is up to 6 cm long and 2 cm thick. Like other bolete mushrooms, S. brevipes produces spores in a vertically arranged layer of spongy tubes with openings that form a layer of small yellowish pores on the underside of the cap.

Suillus spraguei is a species of fungus in the family Suillaceae. It is known by a variety of common names, including the painted slipperycap, the painted suillus or the red and yellow suillus. Suillus spraguei has had a complex taxonomical history, and is also frequently referred to as Suillus pictus in the literature. The readily identifiable fruit bodies have caps that are dark red when fresh, dry to the touch, and covered with mats of hairs and scales that are separated by yellow cracks. On the underside of the cap are small, yellow, angular pores that become brownish as the mushroom ages. The stalk bears a grayish cottony ring, and is typically covered with soft hairs or scales.

Suillus quiescens is a pored mushroom of the genus Suillus in the family Suillaceae. First collected in 2002 on Santa Cruz Island off the coast of California, in association with Bishop Pine, the species was scientifically described and named in 2010. In addition to its distribution in coastal California, it was also found forming ectomycorrhizae with the roots of pine seedlings in the eastern Sierra Nevada, coastal Oregon, and the southern Cascade Mountains. It resembles Suillus brevipes, but can be distinguished from that species by its paler-colored immature cap and by the tiny colored glands on the stipe that darken with age.

Suillus pungens, commonly known as the pungent slippery jack or the pungent suillus, is a species of fungus in the genus Suillus. The fruit bodies of the fungus have slimy convex caps up to 14 cm (5.5 in) wide. The mushroom is characterized by the very distinct color changes that occur in the cap throughout development. Typically, the young cap is whitish, later becoming grayish-olive to reddish-brown or a mottled combination of these colors. The mushroom has a dotted stem (stipe) up to 7 cm (2.8 in) long, and 2 cm (0.8 in) thick. On the underside on the cap is the spore-bearing tissue consisting of minute vertically arranged tubes that appear as a surface of angular, yellowish pores. The presence of milky droplets on the pore surface of young individuals, especially in humid environments, is a characteristic feature of this species. S. pungens can usually be distinguished from other similar Suillus species by differences in distribution, odor and taste. The mushroom is considered edible, but not highly regarded.

Suillus collinitus is a pored mushroom of the genus Suillus in the family Suillaceae. It is an edible mushroom found in European pine forests. The mushroom has a reddish to chestnut-brown cap that reaches up to 11 cm (4.3 in) in diameter, and a yellow stem measuring up to 7 cm (2.8 in) tall by 1 to 2 cm thick. On the underside of the cap are small angular pores, initially bright yellow before turning greenish-brown with age. A characteristic feature that helps to distinguish it from similar Suillus species, such as S. granulatus, is the pinkish mycelia at the base of the stem.
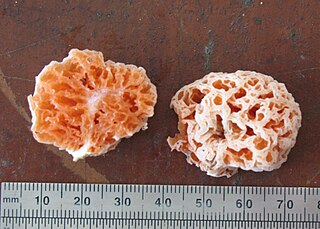
Spongiforma is a genus of sponge-like fungi in the family Boletaceae. Newly described in 2009, the genus contains two species: S. thailandica and S. squarepantsii. The type species S. thailandica is known only from Khao Yai National Park in central Thailand, where it grows in soil in old-growth forests dominated by dipterocarp trees. The rubbery fruit bodies, which has a strong odour of coal-tar similar to Tricholoma sulphureum, consists of numerous internal cavities lined with spore-producing tissue. S. squarepantsii, described as new to science in 2011, is found in Malaysia. It produces sponge-like, rubbery orange fruit bodies with a fruity or musky odour. These fruit bodies will—like a sponge—resume their original shape if water is squeezed out. The origin of the specific name derives from its perceived resemblance to the cartoon character SpongeBob SquarePants. Apart from differences in distribution, S. squarepantsii differs from S. thailandica in its colour, odour, and spore structure.

An ectomycorrhiza is a form of symbiotic relationship that occurs between a fungal symbiont, or mycobiont, and the roots of various plant species. The mycobiont is often from the phyla Basidiomycota and Ascomycota, and more rarely from the Zygomycota. Ectomycorrhizas form on the roots of around 2% of plant species, usually woody plants, including species from the birch, dipterocarp, myrtle, beech, willow, pine and rose families. Research on ectomycorrhizas is increasingly important in areas such as ecosystem management and restoration, forestry and agriculture.

Ectomycorrhizal extramatrical mycelium is the collection of filamentous fungal hyphae emanating from ectomycorrhizas. It may be composed of fine, hydrophilic hypha which branches frequently to explore and exploit the soil matrix or may aggregate to form rhizomorphs; highly differentiated, hydrophobic, enduring, transport structures.

Rhizopogon occidentalis is an ectomycorrhizal fungus in the family Rhizopogonaceae of the Basidiomycota. It occurs most commonly in western North America in association with two-needle and three-needle pine hosts. They are false truffles with fruiting bodies that are yellow on the surface and pale yellow inside. Their edibility is disputed.

Mycorrhiza helper bacteria (MHB) are a group of organisms that form symbiotic associations with both ectomycorrhiza and arbuscular mycorrhiza. MHBs are diverse and belong to a wide variety of bacterial phyla including both Gram-negative and Gram-positive bacteria. Some of the most common MHBs observed in studies belong to the phylas Pseudomonas and Streptomyces. MHBs have been seen to have extremely specific interactions with their fungal hosts at times, but this specificity is lost with plants. MHBs enhance mycorrhizal function, growth, nutrient uptake to the fungus and plant, improve soil conductance, aid against certain pathogens, and help promote defense mechanisms. These bacteria are naturally present in the soil, and form these complex interactions with fungi as plant root development starts to take shape. The mechanisms through which these interactions take shape are not well-understood and needs further study.

Rhizopogon salebrosus is a mushroom species within the Rhizopogon subgenus Amylopogon. R.salebrosus is a monotropoid mycorrhiza that is of vital importance to the ecology of conifer forests, especially in the Pacific Northwest region of North America. Although it is native to North America, R. salebrosus has been found in Europe and its range is generally limited to mountainous regions with sufficient precipitation. The mycoheterotrophic plant, Pterospora andromedea is often found in an obligate association with R. salebrosus in western parts of the U.S. Eastern populations of P. andromedea are typically symbiotic with another Rhizopogon sub species, R. kretzerae.






















