
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell (cytotoxicity) or an organ such as the liver (hepatotoxicity). Sometimes the word is more or less synonymous with poisoning in everyday usage.
Risk assessment determines possible mishaps, their likelihood and consequences, and the tolerances for such events. The results of this process may be expressed in a quantitative or qualitative fashion. Risk assessment is an inherent part of a broader risk management strategy to help reduce any potential risk-related consequences.

Vinyl chloride is an organochloride with the formula H2C=CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer, poly(vinyl chloride) (PVC). Vinyl chloride monomer is among the top twenty largest petrochemicals (petroleum-derived chemicals) in world production. The United States remains the largest vinyl chloride manufacturing region because of its low-production-cost position in chlorine and ethylene raw materials. China is also a large manufacturer and one of the largest consumers of vinyl chloride. Vinyl chloride is a flammable gas that has a sweet odor and is carcinogenic. It can be formed in the environment when soil organisms break down chlorinated solvents. Vinyl chloride that is released by industries or formed by the breakdown of other chlorinated chemicals can enter the air and drinking water supplies. Vinyl chloride is a common contaminant found near landfills. Before the 1970s, vinyl chloride was used as an aerosol propellant and refrigerant.

The Agency for Toxic Substances and Disease Registry (ATSDR) is a federal public health agency within the United States Department of Health and Human Services. The agency focuses on minimizing human health risks associated with exposure to hazardous substances. It works closely with other federal, state, and local agencies; tribal governments; local communities; and healthcare providers. Its mission is to "Serve the public through responsive public health actions to promote healthy and safe environments and prevent harmful exposures." ATSDR was created as an advisory, nonregulatory agency by the Superfund legislation and was formally organized in 1985.

Occupational hygiene is the anticipation, recognition, evaluation, control, and confirmation (ARECC) of protection from risks associated with exposures to hazards in, or arising from, the workplace that may result in injury, illness, impairment, or affect the well-being of workers and members of the community. These hazards or stressors are typically divided into the categories biological, chemical, physical, ergonomic and psychosocial. The risk of a health effect from a given stressor is a function of the hazard multiplied by the exposure to the individual or group. For chemicals, the hazard can be understood by the dose response profile most often based on toxicological studies or models. Occupational hygienists work closely with toxicologists for understanding chemical hazards, physicists for physical hazards, and physicians and microbiologists for biological hazards. Environmental and occupational hygienists are considered experts in exposure science and exposure risk management. Depending on an individual's type of job, a hygienist will apply their exposure science expertise for the protection of workers, consumers and/or communities.
Acceptable daily intake or ADI is a measure of the amount of a specific substance in food or drinking water that can be ingested (orally) daily over a lifetime without an appreciable health risk. ADIs are expressed usually in milligrams per kilograms of body weight per day.

Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) is a European Union regulation dating from 18 December 2006. REACH addresses the production and use of chemical substances, and their potential impacts on both human health and the environment. Its 849 pages took seven years to pass, and it has been described as the most complex legislation in the Union's history and the most important in 20 years. It is the strictest law to date regulating chemical substances and will affect industries throughout the world. REACH entered into force on 1 June 2007, with a phased implementation over the next decade. The regulation also established the European Chemicals Agency, which manages the technical, scientific and administrative aspects of REACH.

The Toxic Substances Control Act (TSCA) is a United States law, passed by the 94th United States Congress in 1976 and administered by the United States Environmental Protection Agency (EPA), that regulates chemicals not regulated by other U.S. federal statutes, including chemicals already in commerce and the introduction of new chemicals. When the TSCA was put into place, all existing chemicals were considered to be safe for use and subsequently grandfathered in. Its three main objectives are to assess and regulate new commercial chemicals before they enter the market, to regulate chemicals already existing in 1976 that posed an "unreasonable risk of injury to health or the environment", as for example PCBs, lead, mercury and radon, and to regulate these chemicals' distribution and use.

The Environmental Working Group (EWG) is an American activist group that specializes in research and advocacy in the areas of agricultural subsidies, toxic chemicals, drinking water pollutants, and corporate accountability. EWG is a nonprofit organization.
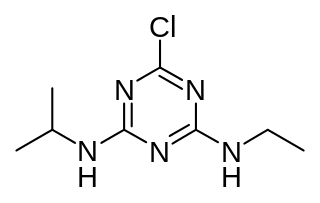
Atrazine is a chlorinated herbicide of the triazine class. It is used to prevent pre-emergence broadleaf weeds in crops such as maize (corn), soybean and sugarcane and on turf, such as golf courses and residential lawns. Atrazine's primary manufacturer is Syngenta and it is one of the most widely used herbicides in the United States, Canadian, and Australian agriculture. Its use was banned in the European Union in 2004, when the EU found groundwater levels exceeding the limits set by regulators, and Syngenta could not show that this could be prevented nor that these levels were safe.

Toxicology testing, also known as safety assessment, or toxicity testing, is the process of determining the degree to which a substance of interest negatively impacts the normal biological functions of an organism, given a certain exposure duration, route of exposure, and substance concentration.
Because of the ongoing controversy on the implications of nanotechnology, there is significant debate concerning whether nanotechnology or nanotechnology-based products merit special government regulation. This mainly relates to when to assess new substances prior to their release into the market, community and environment.
The Hazardous Substances Data Bank (HSDB) was a toxicology database on the U.S. National Library of Medicine's (NLM) Toxicology Data Network (TOXNET). It focused on the toxicology of potentially hazardous chemicals, and included information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, and related areas. All data were referenced and derived from a core set of books, government documents, technical reports, and selected primary journal literature. Prior to 2020, all entries were peer-reviewed by a Scientific Review Panel (SRP), members of which represented a spectrum of professions and interests. Last Chairs of the SRP are Dr. Marcel J. Cassavant, MD, Toxicology Group, and Dr. Roland Everett Langford, PhD, Environmental Fate Group. The SRP was terminated due to budget cuts and realignment of the NLM.

Pesticide regulation in the United States is primarily a responsibility of the Environmental Protection Agency (EPA). In America, it was not till the 1950s that pesticides were regulated in terms of their safety. The Pesticides Control Amendment (PCA) of 1954 was the first time Congress passed guidance regarding the establishment of safe limits for pesticide residues on food. It authorized the Food and Drug Administration (FDA) to ban pesticides they determined to be unsafe if they were sprayed directly on food. The Food Additives Amendment, which included the Delaney Clause, prohibited the pesticide residues from any carcinogenic pesticides in processed food. In 1959, pesticides were required to be registered.
Tissue residue is the concentration of a chemical or compound in an organism's tissue or in a portion of an organism's tissue. Tissue residue is used in aquatic toxicology to help determine the fate of chemicals in aquatic systems, bioaccumulation of a substance, or bioavailability of a substance, account for multiple routes of exposure, and address an organism's exposure to chemical mixtures. A tissue residue approach to toxicity testing is considered a more direct and less variable measure of chemical exposure and is less dependent on external environmental factors than measuring the concentration of a chemical in the exposure media.
The discipline of evidence-based toxicology (EBT) strives to transparently, consistently, and objectively assess available scientific evidence in order to answer questions in toxicology, the study of the adverse effects of chemical, physical, or biological agents on living organisms and the environment, including the prevention and amelioration of such effects. EBT has the potential to address concerns in the toxicological community about the limitations of current approaches to assessing the state of the science. These include concerns related to transparency in decision making, synthesis of different types of evidence, and the assessment of bias and credibility. Evidence-based toxicology has its roots in the larger movement towards evidence-based practices.

An adverse outcome pathway (AOP) is structured representation of biological events leading to adverse effects and is considered relevant to risk assessment. The AOP links in a linear way existing knowledge along one or more series of causally connected key events (KE) between two points — a molecular initiating event (MIE) and an adverse outcome (AO) that occur at a level of biological organization relevant to risk assessment. The linkage between the events is described by key event relationships (KER) that describe the causal relationships between the key events.

Michael L. Dourson is an American toxicologist and Director of Science at the nonprofit organization, Toxicology Excellence for Risk Assessment. He was formerly a senior advisor to the Administrator of EPA, and prior to that, a professor at the Risk Science Center at the University of Cincinnati College of Medicine. Prior to joining the University of Cincinnati, he was founder and president of the nonprofit Toxicology Excellence for Risk Assessment. Earlier in his career, he was employed by the Environmental Protection Agency Environmental Criteria and Assessment Office, among other assignments.
Dennis J. Paustenbach PhD, CIH, DABT, is an American scientist, businessman, researcher, and author. Dennis is currently President of Paustenbach and Associates, which is a consulting firm who uses risk assessment techniques to characterize occupational and environmental health hazards. He is the founder and former president of ChemRisk, a consulting firm specializing in the use of toxicology and risk assessment to characterize the hazards of chemicals in soil, air, water, food, sediments and consumer products. He was, for about 4 years, a Group Vice-President of Exponent.
Threshold dose is the minimum dose of drug that triggers minimal detectable biological effect in an animal. At extremely low doses, biological responses are absent for some of the drugs. The increase in dose above threshold dose induces an increase in the percentage of biological responses. Several benchmarks have been established to describe the effects of a particular dose of drug in a particular species, such as NOEL(no-observed-effect-level), NOAEL(no-observed-adverse-effect-level) and LOAEL(lowest-observed-adverse-effect-level). They are established by reviewing the available studies and animal studies. The application of threshold dose in risk assessment safeguards the participants in human clinical trials and evaluates the risks of chronic exposure to certain substances. However, the nature of animal studies also limits the applicability of experimental results in the human population and its significance in evaluating potential risk of certain substances. In toxicology, there are some other safety factors including LD50, LC50 and EC50.











