Related Research Articles
Dynamic nuclear polarization (DNP) results from transferring spin polarization from electrons to nuclei, thereby aligning the nuclear spins to the extent that electron spins are aligned. Note that the alignment of electron spins at a given magnetic field and temperature is described by the Boltzmann distribution under the thermal equilibrium. It is also possible that those electrons are aligned to a higher degree of order by other preparations of electron spin order such as: chemical reactions, optical pumping and spin injection. DNP is considered one of several techniques for hyperpolarization. DNP can also be induced using unpaired electrons produced by radiation damage in solids.
Microwave spectroscopy is the spectroscopy method that employs microwaves, i.e. electromagnetic radiation at GHz frequencies, for the study of matter.

Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spins excited are those of the electrons instead of the atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes and organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford.

Spin trapping is an analytical technique employed in chemistry and biology for the detection and identification of short-lived free radicals through the use of electron paramagnetic resonance (EPR) spectroscopy. EPR spectroscopy detects paramagnetic species such as the unpaired electrons of free radicals. However, when the half-life of radicals is too short to detect with EPR, compounds known as spin traps are used to react covalently with the radical products and form more stable adduct that will also have paramagnetic resonance spectra detectable by EPR spectroscopy. The use of radical-addition reactions to detect short-lived radicals was developed by several independent groups by 1968.

DPPH is a common abbreviation for the organic chemical compound 2,2-diphenyl-1-picrylhydrazyl. It is a dark-colored crystalline powder composed of stable free radical molecules. DPPH has two major applications, both in laboratory research: one is a monitor of chemical reactions involving radicals, most notably it is a common antioxidant assay, and another is a standard of the position and intensity of electron paramagnetic resonance signals.
Alan Mark Portis was an American solid-state physicist and founder of the Berkeley Physics Laboratory.
Electron nuclear double resonance (ENDOR) is a magnetic resonance technique for elucidating the molecular and electronic structure of paramagnetic species. The technique was first introduced to resolve interactions in electron paramagnetic resonance (EPR) spectra. It is currently practiced in a variety of modalities, mainly in the areas of biophysics and heterogeneous catalysis.
Acoustic paramagnetic resonance (APR) is a phenomenon of resonant absorption of sound by a system of magnetic particles placed in an external magnetic field. It occurs when the energy of the sound wave quantum becomes equal to the splitting of the energy levels of the particles, the splitting being induced by the magnetic field. APR is a variation of electron paramagnetic resonance (EPR) where the acoustic rather than electromagnetic waves are absorbed by the studied sample. APR was theoretically predicted in 1952, independently by Semen Altshuler and Alfred Kastler, and was experimentally observed by W. G. Proctor and W. H. Tanttila in 1955.

Semyon Alexandrovich Altshuler was a Soviet physicist known for his work in resonance spectroscopy and in particular for theoretical prediction of acoustic paramagnetic resonance in 1952.
William Dale Phillips (1925-1993) was an American chemist, nuclear magnetic resonance spectroscopist, federal science policy advisor and member of the National Academy of Sciences. He was born October 10, 1925, in Kansas City, Missouri and died in St. Louis, Missouri, on December 15, 1993.
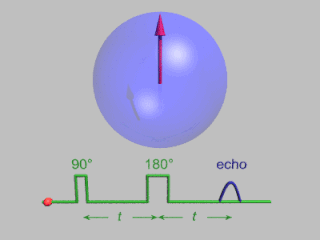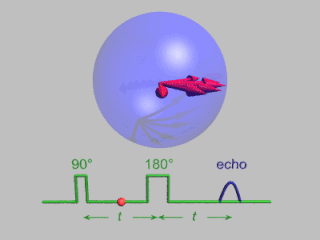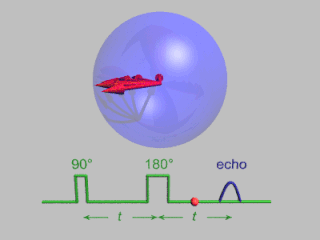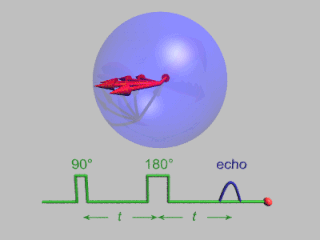
Pulsed electron paramagnetic resonance (EPR) is an electron paramagnetic resonance technique that involves the alignment of the net magnetization vector of the electron spins in a constant magnetic field. This alignment is perturbed by applying a short oscillating field, usually a microwave pulse. One can then measure the emitted microwave signal which is created by the sample magnetization. Fourier transformation of the microwave signal yields an EPR spectrum in the frequency domain. With a vast variety of pulse sequences it is possible to gain extensive knowledge on structural and dynamical properties of paramagnetic compounds. Pulsed EPR techniques such as electron spin echo envelope modulation (ESEEM) or pulsed electron nuclear double resonance (ENDOR) can reveal the interactions of the electron spin with its surrounding nuclear spins.

Paramagnetic nuclear magnetic resonance spectroscopy refers to nuclear magnetic resonance (NMR) spectroscopy of paramagnetic compounds. Although most NMR measurements are conducted on diamagnetic compounds, paramagnetic samples are also amenable to analysis and give rise to special effects indicated by a wide chemical shift range and broadened signals. Paramagnetism diminishes the resolution of an NMR spectrum to the extent that coupling is rarely resolved. Nonetheless spectra of paramagnetic compounds provide insight into the bonding and structure of the sample. For example, the broadening of signals is compensated in part by the wide chemical shift range (often 200 ppm in 1H NMR). Since paramagnetism leads to shorter relaxation times (T1), the rate of spectral acquisition can be high.
James S. Hyde was an American biophysicist. He held the James S. Hyde chair in Biophysics at the Medical College of Wisconsin (MCW) where he specialized in magnetic resonance instrumentation and methodology development in two distinct areas: electron paramagnetic resonance (EPR) spectroscopy and magnetic resonance imaging (MRI). He is senior author of the widely cited 1995 paper by B.B. Biswal et al. reporting the discovery of resting state functional connectivity (fcMRI) in the human brain. He also served as Director of the National Biomedical EPR Center, a Research Resource supported by the National Institutes of Health. He was author of more than 400 peer-reviewed papers and review articles and held 35 U.S. Patents. He was recognized by Festschrifts in both EPR and fcMRI.

Boris Ivanovich Kochelaev is a Soviet and Russian physicist and professor.

Wolfgang Lubitz is a German chemist and biophysicist. He is currently a director emeritus at the Max Planck Institute for Chemical Energy Conversion. He is well known for his work on bacterial photosynthetic reaction centres, hydrogenase enzymes, and the oxygen-evolving complex using a variety of biophysical techniques. He has been recognized by a Festschrift for his contributions to electron paramagnetic resonance (EPR) and its applications to chemical and biological systems.
David Collison is a British chemist and a Professor in the Department of Chemistry at The University of Manchester. His research in general is based on inorganic chemistry and magnetochemistry, specifically on coordination chemistry, electron paramagnetic resonance spectroscopy and f-block chemistry.
R. David Britt is the Winston Ko Chair and Distinguished Professor of Chemistry at the University of California, Davis. Britt uses electron paramagnetic resonance (EPR) spectroscopy to study metalloenzymes and enzymes containing organic radicals in their active sites. Britt is the recipient of multiple awards for his research, including the Bioinorganic Chemistry Award in 2019 and the Bruker Prize in 2015 from the Royal Society of Chemistry. He has received a Gold Medal from the International EPR Society (2014), and the Zavoisky Award from the Kazan Scientific Center of the Russian Academy of Sciences (2018). He is a Fellow of the American Association for the Advancement of Science and of the Royal Society of Chemistry.

Daniella Goldfarb is an Israeli chemist who is the Erich Klieger Professorial Chair in Chemical Physics at the Weizmann Institute of Science. She is the President’s Advisor for Advancing Women in Science. Her research makes use of electron paramagnetic resonance spectroscopy. She was awarded the 2016 Israel Chemical Society Excellence prize.
{{Infobox scientist | name = Marina Bennati | workplaces = [[Max Planck Institute for multidisciplinary Sciences]
[University of Göttingen]]
Goethe University Frankfurt
Massachusetts Institute of Technology | alma_mater = University of Stuttgart
University of Münster | thesis_title = Zeitaufgelöste Elektronen-Spin-Resonanz an photoangeregten Zuständen spezieller Donor-Akzeptor-Systeme | thesis_url = http://www.worldcat.org/oclc/258062810 | thesis_year = 1995 }}
Electron resonance imaging (ERI) is a preclinical imaging method, together with positron emission tomography (PET), computed tomography scan, magnetic resonance imaging (MRI), and other techniques. ERI is dedicated to imaging small laboratory animals and its unique feature is the ability to detect free radicals. This technique could also be used for other purposes such as material science, quality of food, etc.
References
- 1 2 3 4 5 6 7 8 "DU Portfolio". portfolio.du.edu. Retrieved 2018-03-26.
- ↑ Denver, University of. "EPR Center | Natural Sciences & Mathematics | University of Denver". epr-center.du.edu. Retrieved 2018-03-26.
- ↑ Sato, H.; Kathirvelu, V.; Fielding, A.; Blinco, J. P.; Micallef, A. S.; Bottle, S. E.; Eaton, S. S.; Eaton, G. R. (August 2007). "Impact of molecular size on electron spin relaxation rates of nitroxyl radicals in glassy solvents between 100 and 300 K" (PDF). Molecular Physics. 105 (15–16): 2137–2151. Bibcode:2007MolPh.105.2137S. doi:10.1080/00268970701724966. ISSN 0026-8976. S2CID 96023757.
- ↑ Biller, Joshua R.; Meyer, Virginia M.; Elajaili, Hanan; Rosen, Gerald M.; Eaton, Sandra S.; Eaton, Gareth R. (2012). "Frequency dependence of electron spin relaxation times in aqueous solution for a nitronyl nitroxide radical and perdeuterated-tempone between 250MHz and 34GHz". Journal of Magnetic Resonance. 225: 52–57. Bibcode:2012JMagR.225...52B. doi:10.1016/j.jmr.2012.10.002. PMC 3538045 . PMID 23123770.
- ↑ Eaton, Sandra S.; Huber, Kirby; Elajaili, Hanan; McPeak, Joseph; Eaton, Gareth R.; Longobardi, Lauren E.; Stephan, Douglas W. (2017). "Electron spin relaxation of a boron-containing heterocyclic radical". Journal of Magnetic Resonance. 276: 7–13. Bibcode:2017JMagR.276....7E. doi:10.1016/j.jmr.2016.12.013. PMID 28081476.
- ↑ EPR imaging and in vivo EPR. Eaton, Gareth R., Eaton, Sandra S., Ohno, Keiichi. Boca Raton, FL. 29 November 2017. ISBN 9781315892788. OCLC 1020789984.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ↑ R., Eaton, Gareth (1998). Foundations of modern EPR. Eaton, Sandra S., Salikhov, K. M. (Kev Minullinovich). Singapore: World Scientific. ISBN 9789810232955. OCLC 37695761.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ J., Berliner, Lawrence (2002). Distance Measurements in Biological Systems by EPR. Eaton, Gareth R., Eaton, Sandra S. Boston, MA: Springer US. ISBN 9780306465338. OCLC 853270666.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Biomedical EPR. Eaton, Sandra S., Eaton, Gareth R., Berliner, Lawrence J. New York: Kluwer Academic/Plenum Publishers. 2005. ISBN 9780306485060. OCLC 209817713.
{{cite book}}: CS1 maint: others (link) - ↑ Biomedical EPR. Eaton, Sandra S., Eaton, Gareth R., Berliner, Lawrence J. New York: Kluwer Academic/Plenum Publishers. 2005. ISBN 9780306485329. OCLC 209817713.
{{cite book}}: CS1 maint: others (link) - ↑ Quantitative EPR. Eaton, Gareth R., Eaton, Sandra S., Barr, David P., Weber, Ralph Thomas, 1958-. Wien: Springer. 2010. ISBN 9783211929483. OCLC 663098006.
{{cite book}}: CS1 maint: others (link) - ↑ "Making Innovation at DU Available to the World" . Retrieved 2018-03-26.
- ↑ "Real-Time Measurements of Oxygen in a Living Body" . Retrieved 2018-03-26.
- ↑ University of Denver (2016-10-17), Knoebel Institute for Healthy Aging: Sandra and Gareth Eaton , retrieved 2018-03-26
- ↑ "NSF Award Search: Award#1227992 – MRI: Development of an Innovative EPR Spectrometer". www.nsf.gov. Retrieved 2018-03-26.
- ↑ "Gareth and Sandra Eaton Book Fund – Giving to the Library – Harvard College Library". hcl.harvard.edu. Retrieved 2018-03-26.
- ↑ "Sandra Eaton – Google Scholar Citations". scholar.google.com. Retrieved 2018-03-29.
- ↑ "Bruker Lectures | RSC ESR Spectroscopy Group". www.esr-group.org. Retrieved 2018-03-26.