In chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.

Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkaline, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.

Waxes are a diverse class of organic compounds that are lipophilic, malleable solids near ambient temperatures. They include higher alkanes and lipids, typically with melting points above about 40 °C (104 °F), melting to give low viscosity liquids. Waxes are insoluble in water but soluble in organic, nonpolar solvents. Natural waxes of different types are produced by plants and animals and occur in petroleum.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.
Ethoxylation is a chemical reaction in which ethylene oxide adds to a substrate. It is the most widely practiced alkoxylation, which involves the addition of epoxides to substrates.
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention: Production capacity reached 6.6×106 tons in 1995. It is important because aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in speciality chemicals, relevant to the organic synthesis of fragrances and drugs. The development of hydroformylation is one of the premier achievements of 20th-century industrial chemistry.
In chemistry, homogeneous catalysis is catalysis by a soluble catalyst in a solution. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in distinct phases, typically solid-gas, respectively. The term is used almost exclusively to describe solutions and implies catalysis by organometallic compounds. Homogeneous catalysis is established technology that continues to evolve. An illustrative major application is the production of acetic acid. Enzymes are examples of homogeneous catalysts.
Fatty alcohols (or long-chain alcohols) are usually high-molecular-weight, straight-chain primary alcohols, but can also range from as few as 4–6 carbons to as many as 22–26, derived from natural fats and oils. The precise chain length varies with the source. Some commercially important fatty alcohols are lauryl, stearyl, and oleyl alcohols. They are colourless oily liquids (for smaller carbon numbers) or waxy solids, although impure samples may appear yellow. Fatty alcohols usually have an even number of carbon atoms and a single alcohol group (–OH) attached to the terminal carbon. Some are unsaturated and some are branched. They are widely used in industry. As with fatty acids, they are often referred to generically by the number of carbon atoms in the molecule, such as "a C12 alcohol", that is an alcohol having 12 carbons, for example dodecanol.

Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often creates fewer undesired by-products and hazardous wastes than alternative organic reactions. For their elucidation of the reaction mechanism and their discovery of a variety of highly active catalysts, Yves Chauvin, Robert H. Grubbs, and Richard R. Schrock were collectively awarded the 2005 Nobel Prize in Chemistry.

1-Hexene (hex-1-ene) is an organic compound with the formula C6H12. It is an alkene that is classified in industry as higher olefin and an alpha-olefin, the latter term meaning that the double bond is located at the alpha (primary) position, endowing the compound with higher reactivity and thus useful chemical properties. 1-Hexene is an industrially significant linear alpha olefin. 1-Hexene is a colourless liquid.

Alpha-olefins are a family of organic compounds which are alkenes with a chemical formula CxH2x, distinguished by having a double bond at the primary or alpha (α) position. This location of a double bond enhances the reactivity of the compound and makes it useful for a number of applications.

Linear alpha olefins (LAO) or normal alpha olefins (NAO) are olefins or alkenes with a chemical formula CxH2x, distinguished from other mono-olefins with a similar molecular formula by linearity of the hydrocarbon chain and the position of the double bond at the primary or alpha position.

1-Octene is an organic compound with a formula CH2CHC6H13. The alkene is classified as a higher olefin and alpha-olefin, meaning that the double bond is located at the alpha (primary) position, endowing this compound with higher reactivity and thus useful chemical properties. 1-Octene is one of the important linear alpha olefins in industry. It is a colourless liquid.
Decene is an alkene with the formula C
10H
20. Decene contains a chain of ten carbon atoms with one double bond. There are many isomers of decene depending on the position and geometry of the double bond. Dec-1-ene is the only isomer of industrial importance. As an alpha olefin, it is used as a comonomer in copolymers and is an intermediate in the production of epoxides, amines, oxo alcohols, synthetic lubricants, synthetic fatty acids and alkylated aromatics.
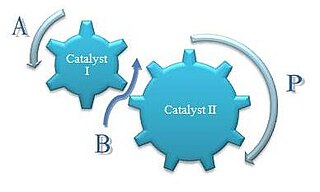
Concurrent tandem catalysis (CTC) is a technique in chemistry where multiple catalysts produce a product otherwise not accessible by a single catalyst. It is usually practiced as homogeneous catalysis. Scheme 1 illustrates this process. Molecule A enters this catalytic system to produce the comonomer, B, which along with A enters the next catalytic process to produce the final product, P. This one-pot approach can decrease product loss from isolation or purification of intermediates. Reactions with relatively unstable products can be generated as intermediates because they are only transient species and are immediately used in a consecutive reaction.
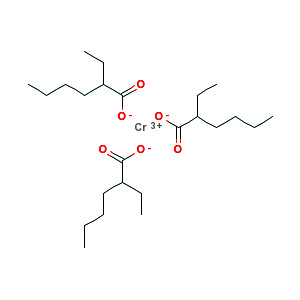
Chromium(III) 2-ethylhexanoate, C24H45CrO6, is a coordination complex of chromium and ethylhexanoate. In combination with 2,5-dimethylpyrrole it forms the Phillips selective ethylene trimerisation catalyst (not to be confused with Phillips catalyst), used in the industrial production of linear alpha olefins, particularly 1-hexene or 1-octene.
Ethenolysis is a chemical process in which internal olefins are degraded using ethylene as the reagent. The reaction is an example of cross metathesis. The utility of the reaction is driven by the low cost of ethylene as a reagent and its selectivity. It produces compounds with terminal alkene functional group (α-olefins), which are more amenable to other reactions such as polymerization and hydroformylation.
In organic chemistry, the Ziegler process is a method for producing fatty alcohols from ethylene using an organoaluminium compound. The reaction produces linear primary alcohols with an even numbered carbon chain. The process uses an aluminum compound to oligomerize ethylene and allow the resulting alkyl group to be oxygenated. The usually targeted products are fatty alcohols, which are otherwise derived from natural fats and oils. Fatty alcohols are used in food and chemical processing. They are useful due to their amphipathic nature. The synthesis route is named after Karl Ziegler, who described the process in 1955.

Herbert S. Eleuterio is an American industrial chemist noted for technical contributions to catalysis, polymerization, industrial research management, and science education. In particular, he discovered the olefin metathesis reaction and several novel fluoropolymers. Additionally, he explored techniques for research leadership, especially methods for fostering collaboration, globalization, and scientific creativity.
Olefin Conversion Technology, also called the Phillips Triolefin Process, is the industrial process that interconverts propylene with ethylene and 2-butenes. The process is also called the ethylene to propylene (ETP) process. In ETP, ethylene is dimerized to 1-butene, which is isomerized to 2-butenes. The 2-butenes are then subjected to metathesis with ethylene.