
An axon, or nerve fiber, is a long, slender projection of a nerve cell, or neuron, in vertebrates, that typically conducts electrical impulses known as action potentials away from the nerve cell body. The function of the axon is to transmit information to different neurons, muscles, and glands. In certain sensory neurons, such as those for touch and warmth, the axons are called afferent nerve fibers and the electrical impulse travels along these from the periphery to the cell body and from the cell body to the spinal cord along another branch of the same axon. Axon dysfunction can be the cause of many inherited and acquired neurological disorders that affect both the peripheral and central neurons. Nerve fibers are classed into three types – group A nerve fibers, group B nerve fibers, and group C nerve fibers. Groups A and B are myelinated, and group C are unmyelinated. These groups include both sensory fibers and motor fibers. Another classification groups only the sensory fibers as Type I, Type II, Type III, and Type IV.
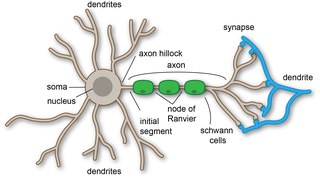
Dendrites, also dendrons, are branched protoplasmic extensions of a nerve cell that propagate the electrochemical stimulation received from other neural cells to the cell body, or soma, of the neuron from which the dendrites project. Electrical stimulation is transmitted onto dendrites by upstream neurons via synapses which are located at various points throughout the dendritic tree.

A neuron, neurone, or nerve cell is an electrically excitable cell that communicates with other cells via specialized connections called synapses. The neuron is the main component of nervous tissue in all animals except sponges and placozoa. Non-animals like plants and fungi do not have nerve cells.
An artificial neuron is a mathematical function conceived as a model of biological neurons, a neural network. Artificial neurons are elementary units in an artificial neural network. The artificial neuron receives one or more inputs and sums them to produce an output. Usually each input is separately weighted, and the sum is passed through a non-linear function known as an activation function or transfer function. The transfer functions usually have a sigmoid shape, but they may also take the form of other non-linear functions, piecewise linear functions, or step functions. They are also often monotonically increasing, continuous, differentiable and bounded. Non-monotonic, unbounded and oscillating activation functions with multiple zeros that outperform sigmoidal and ReLU like activation functions on many tasks have also been recently explored. The thresholding function has inspired building logic gates referred to as threshold logic; applicable to building logic circuits resembling brain processing. For example, new devices such as memristors have been extensively used to develop such logic in recent times.

Pyramidal cells, or pyramidal neurons, are a type of multipolar neuron found in areas of the brain including the cerebral cortex, the hippocampus, and the amygdala. Pyramidal neurons are the primary excitation units of the mammalian prefrontal cortex and the corticospinal tract. Pyramidal neurons are also one of two cell types where the characteristic sign, Negri bodies, are found in post-mortem rabies infection. Pyramidal neurons were first discovered and studied by Santiago Ramón y Cajal. Since then, studies on pyramidal neurons have focused on topics ranging from neuroplasticity to cognition.
An apical dendrite is a dendrite that emerges from the apex of a pyramidal cell. Apical dendrites are one of two primary categories of dendrites, and they distinguish the pyramidal cells from spiny stellate cells in the cortices. Pyramidal cells are found in the prefrontal cortex, the hippocampus, the entorhinal cortex, the olfactory cortex, and other areas. Dendrite arbors formed by apical dendrites are the means by which synaptic inputs into a cell are integrated. The apical dendrites in these regions contribute significantly to memory, learning, and sensory associations by modulating the excitatory and inhibitory signals received by the pyramidal cells.
NeuronStudio was a non-commercial program created at Icahn School of Medicine at Mount Sinai by the Computational Neurobiology and Imaging Center. This program performed automatic tracing and reconstruction of neuron structures from confocal image stacks. The resulting models were then be exported to file using standard formats for further processing, modeling, or for statistical analyses. NeuronStudio handled morphologic details on scales spanning local Dendritic spine geometry through complex tree topology to the gross spatial arrangement of multi-neuron networks. Its capability for automated digitization avoided the subjective errors inherent in manual tracing. The program ceased to be supported in 2012 and the project pages were eventually removed from the ISMMS Website. Its documentation and the Windows source code however are still available via the Internet Archive.
A neurite or neuronal process refers to any projection from the cell body of a neuron. This projection can be either an axon or a dendrite. The term is frequently used when speaking of immature or developing neurons, especially of cells in culture, because it can be difficult to tell axons from dendrites before differentiation is complete.

A unipolar neuron is a neuron in which only one process, called a neurite, extends from the cell body. The neurite then branches to form dendritic and axonal processes. Most neurons in the central nervous systems of invertebrates, including insects, are unipolar. The cell bodies of invertebrate unipolar neurons are often located around the edges of the neuropil, in the so-called cell-body rind.

Classical cable theory uses mathematical models to calculate the electric current along passive neurites, particularly the dendrites that receive synaptic inputs at different sites and times. Estimates are made by modeling dendrites and axons as cylinders composed of segments with capacitances and resistances combined in parallel. The capacitance of a neuronal fiber comes about because electrostatic forces are acting through the very thin lipid bilayer. The resistance in series along the fiber is due to the axoplasm's significant resistance to movement of electric charge.
Neuromorphology is the study of nervous system form, shape, and structure. The study involves looking at a particular part of the nervous system from a molecular and cellular level and connecting it to a physiological and anatomical point of view. The field also explores the communications and interactions within and between each specialized section of the nervous system. Morphology is distinct from morphogenesis. Morphology is the study of the shape and structure of biological organisms, while morphogenesis is the study of the biological development of the shape and structure of organisms. Therefore, neuromorphology focuses on the specifics of the structure of the nervous system and not the process by which the structure was developed. Neuromorphology and morphogenesis, while two different entities, are nonetheless closely linked.
The synaptotropic hypothesis, also called the synaptotrophic hypothesis, is a neurobiological hypothesis of neuronal growth and synapse formation. The hypothesis was first formulated by J.E. Vaughn in 1988, and remains a focus of current research efforts. The synaptotropic hypothesis proposes that input from a presynaptic to a postsynaptic cell eventually can change the course of synapse formation at dendritic and axonal arbors. This synapse formation is required for the development of neuronal structure in the functioning brain.
Neural backpropagation is the phenomenon in which, after the action potential of a neuron creates a voltage spike down the axon, another impulse is generated from the soma and propagates towards the apical portions of the dendritic arbor or dendrites. In addition to active backpropagation of the action potential, there is also passive electrotonic spread. While there is ample evidence to prove the existence of backpropagating action potentials, the function of such action potentials and the extent to which they invade the most distal dendrites remain highly controversial.

Biological neuron models, also known as a spiking neuron models, are mathematical descriptions of the properties of certain cells in the nervous system that generate sharp electrical potentials across their cell membrane, roughly one millisecond in duration, called action potentials or spikes. Since spikes are transmitted along the axon and synapses from the sending neuron to many other neurons, spiking neurons are considered to be a major information processing unit of the nervous system. Spiking neuron models can be divided into different categories: the most detailed mathematical models are biophysical neuron models that describe the membrane voltage as a function of the input current and the activation of ion channels. Mathematically simpler are integrate-and-fire models that describe the membrane voltage as a function of the input current and predict the spike times without a description of the biophysical processes that shape the time course of an action potential. Even more abstract models only predict output spikes as a function of the stimulation where the stimulation can occur through sensory input or pharmacologically. This article provides a short overview of different spiking neuron models and links, whenever possible to experimental phenomena. It includes deterministic and probabilistic models.

In neurophysiology, a dendritic spike refers to an action potential generated in the dendrite of a neuron. Dendrites are branched extensions of a neuron. They receive electrical signals emitted from projecting neurons and transfer these signals to the cell body, or soma. Dendritic signaling has traditionally been viewed as a passive mode of electrical signaling. Unlike its axon counterpart which can generate signals through action potentials, dendrites were believed to only have the ability to propagate electrical signals by physical means: changes in conductance, length, cross sectional area, etc. However, the existence of dendritic spikes was proposed and demonstrated by W. Alden Spencer, Eric Kandel, Rodolfo Llinás and coworkers in the 1960s and a large body of evidence now makes it clear that dendrites are active neuronal structures. Dendrites contain voltage-gated ion channels giving them the ability to generate action potentials. Dendritic spikes have been recorded in numerous types of neurons in the brain and are thought to have great implications in neuronal communication, memory, and learning. They are one of the major factors in long-term potentiation.
Compartmental modelling of dendrites deals with multi-compartment modelling of the dendrites, to make the understanding of the electrical behavior of complex dendrites easier. Basically, compartmental modelling of dendrites is a very helpful tool to develop new biological neuron models. Dendrites are very important because they occupy the most membrane area in many of the neurons and give the neuron an ability to connect to thousands of other cells. Originally the dendrites were thought to have constant conductance and current but now it has been understood that they may have active Voltage-gated ion channels, which influences the firing properties of the neuron and also the response of neuron to synaptic inputs. Many mathematical models have been developed to understand the electric behavior of the dendrites. Dendrites tend to be very branchy and complex, so the compartmental approach to understand the electrical behavior of the dendrites makes it very useful.
Neuronal tracing, or neuron reconstruction is a technique used in neuroscience to determine the pathway of the neurites or neuronal processes, the axons and dendrites, of a neuron. From a sample preparation point of view, it may refer to some of the following as well as other genetic neuron labeling techniques,
Neuronal tiling is a phenomenon in which multiple arbors of neurons innervate the same surface or tissue in a nonredundant and tiled pattern that maximizes coverage of the surface while minimizing overlap between neighboring arbors. Hence, dendrites of the same neuron spread out by avoiding one another (self-avoidance). Moreover, dendrites of certain types of neurons such as class III and class IV dendritic arborization neurons avoid dendrites of neighboring neurons of the same type (tiling), whereas dendrites of different neuronal types can cover the same territory (coexistence).

Neuronal self-avoidance, or isoneural avoidance, is an important property of neurons which consists in the tendency of branches arising from a single soma to turn away from one another. The arrangements of branches within neuronal arbors are established during development and result in minimal crossing or overlap as they spread over a territory, resulting in the typical fasciculated morphology of neurons.

Michael A. Häusser FRS FMedSci is professor of Neuroscience, based in the Wolfson Institute for Biomedical Research at University College London (UCL).













