In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.
Methylation, in the chemical sciences, is the addition of a methyl group on a substrate, or the substitution of an atom by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These terms are commonly used in chemistry, biochemistry, soil science, and biology.

Potassium ferricyanide is the chemical compound with the formula K3[Fe(CN)6]. This bright red salt contains the octahedrally coordinated [Fe(CN)6]3− ion. It is soluble in water and its solution shows some green-yellow fluorescence. It was discovered in 1822 by Leopold Gmelin.
Transesterification is the process of exchanging the organic functional group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. Strong acids catalyze the reaction by donating a proton to the carbonyl group, thus making it a more potent electrophile. Bases catalyze the reaction by removing a proton from the alcohol, thus making it more nucleophilic. The reaction can also be accomplished with the help of other enzymes, particularly lipases.

Potassium hydroxide is an inorganic compound with the formula KOH, and is commonly called caustic potash.

Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula AlCl3. It forms a hexahydrate with the formula [Al(H2O)6]Cl3, containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour.
The Hofmann rearrangement is the organic reaction of a primary amide to a primary amine with one less carbon atom. The reaction involves oxidation of the nitrogen followed by rearrangement of the carbonyl and nitrogen to give an isocyanate intermediate. The reaction can form a wide range of products, including alkyl and aryl amines.
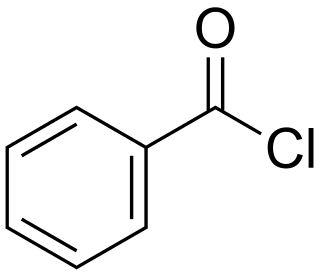
Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula C7H5ClO. It is a colourless, fuming liquid with an irritating odour, and consists of a benzene ring with an acyl chloride substituent. It is mainly useful for the production of peroxides but is generally useful in other areas such as in the preparation of dyes, perfumes, pharmaceuticals, and resins.
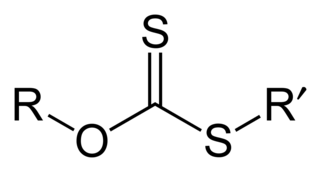
A xanthate is a salt or ester of a xanthic acid. The formula of the salt of xanthic acid is [R−O−CS2]−M+. Xanthate also refers to the anion [R−O−CS2]−. The formula of a xanthic acid is R−O−C(=S)−S−H, such as ethyl xanthic acid, while the formula of an ester of a xanthic acid is R−O−C(=S)−S−R', where R and R' are organyl groups. The salts of xanthates are also called O-organyl dithioates. The esters of xanthic acid are also called O,S-diorganyl esters of dithiocarbonic acid. The name xanthate is derived from Ancient Greek ξανθός (xanthos) meaning 'yellowish' or 'golden', and indeed most xanthate salts are yellow. They were discovered and named in 1823 by Danish chemist William Christopher Zeise. These organosulfur compounds are important in two areas: the production of cellophane and related polymers from cellulose and for extraction of certain sulphide bearing ores. They are also versatile intermediates in organic synthesis.
The Tishchenko reaction is an organic chemical reaction that involves disproportionation of an aldehyde in the presence of an alkoxide. The reaction is named after Russian organic chemist Vyacheslav Tishchenko, who discovered that aluminium alkoxides are effective catalysts for the reaction.
The Bouveault–Blanc reduction is a chemical reaction in which an ester is reduced to primary alcohols using absolute ethanol and sodium metal. It was first reported by Louis Bouveault and Gustave Louis Blanc in 1903. Bouveault and Blanc demonstrated the reduction of ethyl oleate and n-butyl oleate to oleyl alcohol. Modified versions of which were subsequently refined and published in Organic Syntheses.
Sodium ethoxide, also referred to as sodium ethanolate, is the ionic, organic compound with the formula CH3CH2ONa, C2H5ONa, or NaOEt. It is a white solid, although impure samples appear yellow or brown. It dissolves in polar solvents such as ethanol. It is commonly used as a strong base.

The Favorskii rearrangement is principally a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid, but usually either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α'-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds.
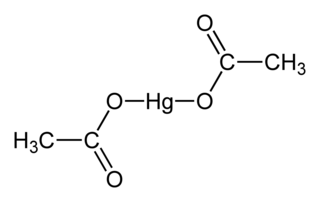
Mercury(II) acetate, also known as mercuric acetate is a chemical compound, the mercury(II) salt of acetic acid, with the formula Hg(O2CCH3)2. Commonly abbreviated Hg(OAc)2, this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors. It is a white, water-soluble solid, but some samples can appear yellowish with time owing to decomposition.

Sodium phenoxide (sodium phenolate) is an organic compound with the formula NaOC6H5. It is a white crystalline solid. Its anion, phenoxide, also known as phenolate, is the conjugate base of phenol. It is used as a precursor to many other organic compounds, such as aryl ethers.
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity.
Potassium methoxide is the alkoxide of methanol with the counterion potassium and is used as a strong base and as a catalyst for transesterification, in particular for the production of biodiesel.

Sodium chloroacetate is the organic compound with the formula CH2ClCO2Na. A white, water-soluble solid, it is the sodium salt of chloroacetic acid. Many of its uses are similar to those of the parent acid. It is prepared by treating chloroacetic acid with sodium carbonate.
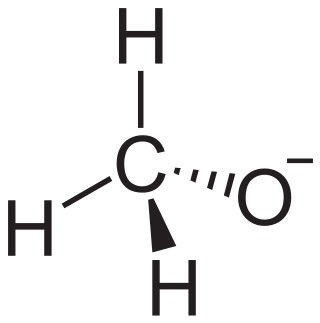
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as RO−, where R is the organyl substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands. Alkoxides, although generally not stable in protic solvents such as water, occur widely as intermediates in various reactions, including the Williamson ether synthesis. Transition metal alkoxides are widely used for coatings and as catalysts.