
In materials science, creep is the tendency of a solid material to undergo slow deformation while subject to persistent mechanical stresses. It can occur as a result of long-term exposure to high levels of stress that are still below the yield strength of the material. Creep is more severe in materials that are subjected to heat for long periods and generally increase as they near their melting point.

In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to slide over each other at low stress levels and is known as glide or slip. The crystalline order is restored on either side of a glide dislocation but the atoms on one side have moved by one position. The crystalline order is not fully restored with a partial dislocation. A dislocation defines the boundary between slipped and unslipped regions of material and as a result, must either form a complete loop, intersect other dislocations or defects, or extend to the edges of the crystal. A dislocation can be characterised by the distance and direction of movement it causes to atoms which is defined by the Burgers vector. Plastic deformation of a material occurs by the creation and movement of many dislocations. The number and arrangement of dislocations influences many of the properties of materials.

In materials science, work hardening, also known as strain hardening, is the strengthening of a metal or polymer by plastic deformation. Work hardening may be desirable, undesirable, or inconsequential, depending on the context.
Precipitation hardening, also called age hardening or particle hardening, is a heat treatment technique used to increase the yield strength of malleable materials, including most structural alloys of aluminium, magnesium, nickel, titanium, and some steels, stainless steels, and duplex stainless steel. In superalloys, it is known to cause yield strength anomaly providing excellent high-temperature strength.

A superalloy, or high-performance alloy, is an alloy with the ability to operate at a high fraction of its melting point. Key characteristics of a superalloy include mechanical strength, thermal creep deformation resistance, surface stability, and corrosion and oxidation resistance.
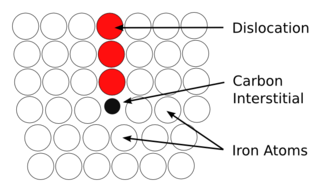
In materials science, the concept of the Cottrell atmosphere was introduced by A. H. Cottrell and B. A. Bilby in 1949 to explain how dislocations are pinned in some metals by boron, carbon, or nitrogen interstitials.
Hardening is a metallurgical metalworking process used to increase the hardness of a metal. The hardness of a metal is directly proportional to the uniaxial yield stress at the location of the imposed strain. A harder metal will have a higher resistance to plastic deformation than a less hard metal.
A solid solution, a term popularly used for metals, is a homogeneous mixture of two different kinds of atoms in solid state and having a single crystal structure. Many examples can be found in metallurgy, geology, and solid-state chemistry. The word "solution" is used to describe the intimate mixing of components at the atomic level and distinguishes these homogeneous materials from physical mixtures of components. Two terms are mainly associated with solid solutions – solvents and solutes, depending on the relative abundance of the atomic species.
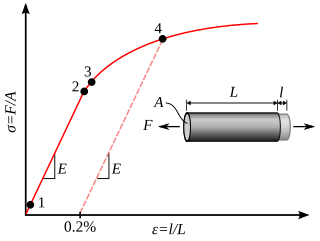
In materials science and engineering, the yield point is the point on a stress-strain curve that indicates the limit of elastic behavior and the beginning of plastic behavior. Below the yield point, a material will deform elastically and will return to its original shape when the applied stress is removed. Once the yield point is passed, some fraction of the deformation will be permanent and non-reversible and is known as plastic deformation.
In materials science, hardness is a measure of the resistance to localized plastic deformation, such as an indentation or a scratch (linear), induced mechanically either by pressing or abrasion. In general, different materials differ in their hardness; for example hard metals such as titanium and beryllium are harder than soft metals such as sodium and metallic tin, or wood and common plastics. Macroscopic hardness is generally characterized by strong intermolecular bonds, but the behavior of solid materials under force is complex; therefore, hardness can be measured in different ways, such as scratch hardness, indentation hardness, and rebound hardness. Hardness is dependent on ductility, elastic stiffness, plasticity, strain, strength, toughness, viscoelasticity, and viscosity. Common examples of hard matter are ceramics, concrete, certain metals, and superhard materials, which can be contrasted with soft matter.

A nanocrystalline (NC) material is a polycrystalline material with a crystallite size of only a few nanometers. These materials fill the gap between amorphous materials without any long range order and conventional coarse-grained materials. Definitions vary, but nanocrystalline material is commonly defined as a crystallite (grain) size below 100 nm. Grain sizes from 100 to 500 nm are typically considered "ultrafine" grains.
Methods have been devised to modify the yield strength, ductility, and toughness of both crystalline and amorphous materials. These strengthening mechanisms give engineers the ability to tailor the mechanical properties of materials to suit a variety of different applications. For example, the favorable properties of steel result from interstitial incorporation of carbon into the iron lattice. Brass, a binary alloy of copper and zinc, has superior mechanical properties compared to its constituent metals due to solution strengthening. Work hardening has also been used for centuries by blacksmiths to introduce dislocations into materials, increasing their yield strengths.

In materials science, grain-boundary strengthening is a method of strengthening materials by changing their average crystallite (grain) size. It is based on the observation that grain boundaries are insurmountable borders for dislocations and that the number of dislocations within a grain has an effect on how stress builds up in the adjacent grain, which will eventually activate dislocation sources and thus enabling deformation in the neighbouring grain as well. By changing grain size, one can influence the number of dislocations piled up at the grain boundary and yield strength. For example, heat treatment after plastic deformation and changing the rate of solidification are ways to alter grain size.
In materials science, segregation is the enrichment of atoms, ions, or molecules at a microscopic region in a materials system. While the terms segregation and adsorption are essentially synonymous, in practice, segregation is often used to describe the partitioning of molecular constituents to defects from solid solutions, whereas adsorption is generally used to describe such partitioning from liquids and gases to surfaces. The molecular-level segregation discussed in this article is distinct from other types of materials phenomena that are often called segregation, such as particle segregation in granular materials, and phase separation or precipitation, wherein molecules are segregated in to macroscopic regions of different compositions. Segregation has many practical consequences, ranging from the formation of soap bubbles, to microstructural engineering in materials science, to the stabilization of colloidal suspensions.
Oxide dispersion strengthened alloys (ODS) are alloys that consist of a metal matrix with small oxide particles dispersed within it. They have high heat resistance, strength, and ductility. Alloys of nickel are the most common but includes iron aluminum alloys.
Dislocation creep is a deformation mechanism in crystalline materials. Dislocation creep involves the movement of dislocations through the crystal lattice of the material, in contrast to diffusion creep, in which diffusion is the dominant creep mechanism. It causes plastic deformation of the individual crystals, and thus the material itself.
An antiphase domain (APD) is a type of planar crystallographic defect in which the atoms within a region of a crystal are configured in the opposite order to those in the perfect lattice system. Throughout the entire APD, atoms sit on the sites typically occupied by atoms of a different species. For example, in an ordered AB alloy, if an A atom occupies the site usually occupied by a B atom, a type of crystallographic point defect called an antisite defect is formed. If an entire region of the crystal is translated such that every atom in a region of the plane of atoms sits on its antisite, an antiphase domain is formed. In other words, an APD is a region formed from antisite defects of a parent lattice. On either side of this domain, the lattice is still perfect, and the boundaries of the domain are referred to as antiphase boundaries. Crucially, crystals on either side of an antiphase boundary are related by a translation, rather than a reflection or an inversion.
Dynamic strain aging (DSA) for materials science is an instability in plastic flow of materials, associated with interaction between moving dislocations and diffusing solutes. Although sometimes dynamic strain aging is used interchangeably with the Portevin–Le Chatelier effect (or serrated yielding), dynamic strain aging refers specifically to the microscopic mechanism that induces the Portevin–Le Chatelier effect. This strengthening mechanism is related to solid-solution strengthening and has been observed in a variety of fcc and bcc substitutional and interstitial alloys, metalloids like silicon, and ordered intermetallics within specific ranges of temperature and strain rate.
Nabarro–Herring creep is a mode of deformation of crystalline materials that occurs at low stresses and held at elevated temperatures in fine-grained materials. In Nabarro–Herring creep, atoms diffuse through the crystals, and the creep rate varies inversely with the square of the grain size so fine-grained materials creep faster than coarser-grained ones. NH creep is solely controlled by diffusional mass transport. This type of creep results from the diffusion of vacancies from regions of high chemical potential at grain boundaries subjected to normal tensile stresses to regions of lower chemical potential where the average tensile stresses across the grain boundaries are zero. Self-diffusion within the grains of a polycrystalline solid can cause the solid to yield to an applied shearing stress, the yielding being caused by a diffusional flow of matter within each crystal grain away from boundaries where there is a normal pressure and toward those where there is a normal tension. Atoms migrating in the opposite direction account for the creep strain. The creep strain rate is derived in the next section. NH creep is more important in ceramics than metals as dislocation motion is more difficult to effect in ceramics.
Anelasticity is a property of materials that describes their behaviour when undergoing deformation. Its formal definition does not include the physical or atomistic mechanisms but still interprets the anelastic behaviour as a manifestation of internal relaxation processes. It is a behaviour differing from elastic behaviour.





















