The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
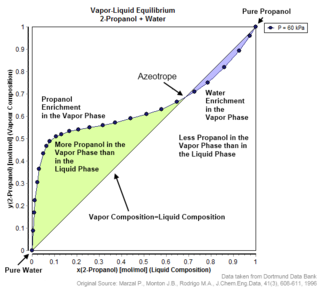
An azeotrope or a constant boiling point mixture is a mixture of two or more liquids whose proportions cannot be altered or changed by simple distillation. This happens because when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Because their composition is unchanged by distillation, azeotropes are also called constant boiling point mixtures.
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (77 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used.
Pervaporation is a processing method for the separation of mixtures of liquids by partial vaporization through a non-porous or porous membrane.

A still is an apparatus used to distill liquid mixtures by heating to selectively boil and then cooling to condense the vapor. A still uses the same concepts as a basic distillation apparatus, but on a much larger scale. Stills have been used to produce perfume and medicine, water for injection (WFI) for pharmaceutical use, generally to separate and purify different chemicals, and to produce distilled beverages containing ethanol.

Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In some situations, GC may help in identifying a compound. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.

Vacuum distillation is a method of distillation performed under reduced pressure. As with distillation, this technique separates compounds based on differences in boiling points. This technique is used when the boiling point of the desired compound is difficult to achieve or will cause the compound to decompose. A reduced pressure decreases the boiling point of compounds. The reduction in boiling point can be calculated using a temperature-pressure nomograph using the Clausius–Clapeyron_relation.
Steam distillation is a special type of distillation for temperature sensitive materials like natural aromatic compounds. It once was a popular laboratory method for purification of organic compounds, but has become less common due to the proliferation of vacuum distillation. Steam distillation remains important in certain industrial sectors.

Liquefaction of gases is physical conversion of a gas into a liquid state (condensation).

Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously fed into the process and separated fractions are removed continuously as output streams. Distillation is the separation or partial separation of a liquid feed mixture into components or fractions by selective boiling and condensation. The process produces at least two output fractions. These fractions include at least one volatile distillate fraction, which has boiled and been separately captured as a vapor condensed to a liquid, and practically always a bottoms fraction, which is the least volatile residue that has not been separately captured as a condensed vapor.
A zeotropicmixture, or non-azeotropic mixture, is a mixture with components that have different boiling points. For example, nitrogen, methane, ethane, propane, and isobutane constitute a zeotropic mixture. Individual substances within the mixture do not evaporate or condense at the same temperature as one substance. In other words, the mixture has a temperature glide, as the phase change occurs in a temperature range of about four to seven degrees Celsius, rather than at a constant temperature. On temperature-composition graphs, this temperature glide can be seen as the temperature difference between the bubble point and dew point. For zeotropic mixtures, the temperatures on the bubble (boiling) curve are between the individual component's boiling temperatures. When a zeotropic mixture is boiled or condensed, the composition of the liquid and the vapor changes according to the mixtures's temperature-composition diagram.
Reboilers are heat exchangers typically used to provide heat to the bottom of industrial distillation columns. They boil the liquid from the bottom of a distillation column to generate vapors which are returned to the column to drive the distillation separation. The heat supplied to the column by the reboiler at the bottom of the column is removed by the condenser at the top of the column.

A Kugelrohr is a short-path vacuum distillation apparatus typically used to distill relatively small amounts of compounds with high boiling points under greatly reduced pressure.
In thermodynamics and chemical engineering, the vapor–liquid equilibrium (VLE) describes the distribution of a chemical species between the vapor phase and a liquid phase.

A condenser is an apparatus or item of equipment used to condense. In the laboratory, condensers are generally used in procedures involving organic liquids brought into the gaseous state through heating, with or without lowering the pressure —though applications in inorganic and other chemistry areas exist. While condensers can be applied at various scales, in the research, training, or discovery laboratory, one most often uses glassware designed to pass a vapor flow over an adjacent cooled chamber. In simplest form, such a condenser consists of a single glass tube with outside air providing cooling. A further simple form, the Liebig-type of condenser, involves concentric glass tubes, an inner one through which the hot gases pass, and an outer, "ported" chamber through which a cooling fluid passes, to reduce the gas temperature in the inner, to afford the condensation.

Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to reactions over a long period of time.
Distillation of crude oil is typically performed either under atmospheric pressure and under a vacuum. Low boiling fractions usually vaporize below 400 °C at atmospheric pressure without cracking the hydrocarbon compounds. Therefore, all the low boiling fractions of crude oil are separated by atmospheric distillation. A crude distillation unit (CDU) consists of pre-flash distillation column. The petroleum products obtained from the distillation process are light, medium, and heavy naphtha, kerosene, diesel, and oil residue.