Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining.

Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other elements, chiefly hydrogen, sulfur, oxygen, and nitrogen. Coal is a type of fossil fuel, formed when dead plant matter decays into peat and is converted into coal by the heat and pressure of deep burial over millions of years. Vast deposits of coal originate in former wetlands called coal forests that covered much of the Earth's tropical land areas during the late Carboniferous (Pennsylvanian) and Permian times.

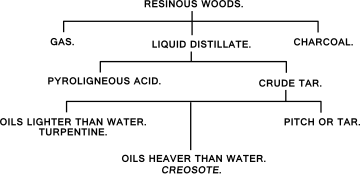
Creosote is a category of carbonaceous chemicals formed by the distillation of various tars and pyrolysis of plant-derived material, such as wood, or fossil fuel. They are typically used as preservatives or antiseptics.

Coke is a grey, hard, and porous coal-based fuel with a high carbon content and few impurities, made by heating coal or oil in the absence of air—a destructive distillation process. It is an important industrial product, used mainly in iron ore smelting, but also as a fuel in stoves and forges when air pollution is a concern.

The pyrolysis process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere.
Coal gas is a flammable gaseous fuel made from coal and supplied to the user via a piped distribution system. It is produced when coal is heated strongly in the absence of air. Town gas is a more general term referring to manufactured gaseous fuels produced for sale to consumers and municipalities.

Industrial processes are procedures involving chemical, physical, electrical, or mechanical steps to aid in the manufacturing of an item or items, usually carried out on a very large scale. Industrial processes are the key components of heavy industry.
Calcination is thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), generally for the purpose of removing impurities or volatile substances and/or to incur thermal decomposition.
In industrial chemistry, coal gasification is the process of producing syngas—a mixture consisting primarily of carbon monoxide (CO), hydrogen, carbon dioxide, methane, and water vapour —from coal and water, air and/or oxygen.
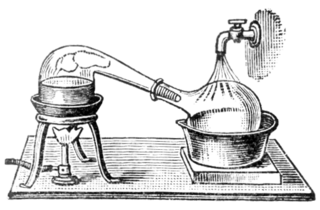
Destructive distillation is a chemical process in which decomposition of unprocessed material is achieved by heating it to a high temperature; the term generally applies to processing of organic material in the absence of air or in the presence of limited amounts of oxygen or other reagents, catalysts, or solvents, such as steam or phenols. It is an application of pyrolysis. The process breaks up or 'cracks' large molecules. Coke, coal gas, gaseous carbon, coal tar, ammonia liquor, and coal oil are examples of commercial products historically produced by the destructive distillation of coal.

Tar is a dark brown or black viscous liquid of hydrocarbons and free carbon, obtained from a wide variety of organic materials through destructive distillation. Tar can be produced from coal, wood, petroleum, or peat.
Carbonization is the conversion of organic matters like plants and dead animal remains into carbon through destructive distillation.
Charring is a chemical process of incomplete combustion of certain solids when subjected to high heat. Heat distillation removes water vapour and volatile organic compounds (syngas) from the matrix. The residual black carbon material is char, as distinguished from the lighter colored ash. By the action of heat, charring removes hydrogen and oxygen from the solid, so that the remaining char is composed primarily of carbon. Polymers like thermoset, or most solid organic compounds like wood or biological tissue, exhibit charring behaviour.
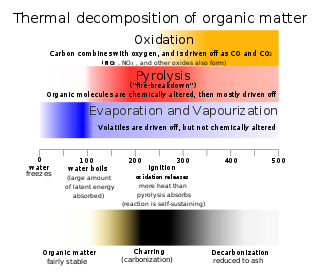
Thermal decomposition is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion or other chemical reaction.

The Karrick process is a low-temperature carbonization (LTC) and pyrolysis process of carbonaceous materials. Although primarily meant for coal carbonization, it also could be used for processing of oil shale, lignite or any carbonaceous materials. These are heated at 450 °C (800 °F) to 700 °C (1,300 °F) in the absence of air to distill out synthetic fuels–unconventional oil and syngas. It could be used for a coal liquefaction as also for a semi-coke production. The process was the work of oil shale technologist Lewis Cass Karrick at the United States Bureau of Mines in the 1920s.
Pyrolysis oil, sometimes also known as bio-crude or bio-oil, is a synthetic fuel with limited industrial application and under investigation as substitute for petroleum. It is obtained by heating dried biomass without oxygen in a reactor at a temperature of about 500 °C (900 °F) with subsequent cooling, separation from the aqueous phase and other processes. Pyrolysis oil is a kind of tar and normally contains levels of oxygen too high to be considered a pure hydrocarbon. This high oxygen content results in non-volatility, corrosiveness, partial miscibility with fossil fuels, thermal instability, and a tendency to polymerize when exposed to air. As such, it is distinctly different from petroleum products. Removing oxygen from bio-oil or nitrogen from algal bio-oil is known as upgrading.

Smokeless fuel is a type of solid fuel which either does not emit visible smoke or emits minimal amounts during combustion. These types of fuel find use where the use of fuels which produce smoke, such as coal and unseasoned or wet wood, is prohibited.

The history of gaseous fuel, important for lighting, heating, and cooking purposes throughout most of the 19th century and the first half of the 20th century, began with the development of analytical and pneumatic chemistry in the 18th century. These "synthetic fuel gases" were made by gasification of combustible materials, usually coal, but also wood and oil, by heating them in enclosed ovens with an oxygen-poor atmosphere. The fuel gases generated were mixtures of many chemical substances, including hydrogen, methane, carbon monoxide and ethylene. Coal gas also contains significant quantities of unwanted sulfur and ammonia compounds, as well as heavy hydrocarbons, and must be purified before use.

Charcoal is a lightweight black carbon residue produced by strongly heating wood in minimal oxygen to remove all water and volatile constituents. In the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is supplied by burning part of the starting material itself, with a limited supply of oxygen. The material can also be heated in a closed retort. Modern "charcoal" briquettes used for outdoor cooking may contain many other additives, e.g. coal.

Hydrothermal carbonization (HTC) is a chemical process for the conversion of organic compounds to structured carbons. It can be used to make a wide variety of nanostructured carbons, simple production of brown coal substitute, synthesis gas, liquid petroleum precursors and humus from biomass with release of energy. Technically the process imitates, within a few hours, the brown coal formation process which takes place in nature over enormously longer geological time periods of 50,000 to 50 million years. It was investigated by Friedrich Bergius and first described in 1913.