
An exon is any part of a gene that will form a part of the final mature RNA produced by that gene after introns have been removed by RNA splicing. The term exon refers to both the DNA sequence within a gene and to the corresponding sequence in RNA transcripts. In RNA splicing, introns are removed and exons are covalently joined to one another as part of generating the mature RNA. Just as the entire set of genes for a species constitutes the genome, the entire set of exons constitutes the exome.
An intron is any nucleotide sequence within a gene that is not expressed or operative in the final RNA product. The word intron is derived from the term intragenic region, i.e., a region inside a gene. The term intron refers to both the DNA sequence within a gene and the corresponding RNA sequence in RNA transcripts. The non-intron sequences that become joined by this RNA processing to form the mature RNA are called exons.

Protein biosynthesis is a core biological process, occurring inside cells, balancing the loss of cellular proteins through the production of new proteins. Proteins perform a number of critical functions as enzymes, structural proteins or hormones. Protein synthesis is a very similar process for both prokaryotes and eukaryotes but there are some distinct differences.

RNA splicing is a process in molecular biology where a newly-made precursor messenger RNA (pre-mRNA) transcript is transformed into a mature messenger RNA (mRNA). It works by removing all the introns and splicing back together exons. For nuclear-encoded genes, splicing occurs in the nucleus either during or immediately after transcription. For those eukaryotic genes that contain introns, splicing is usually needed to create an mRNA molecule that can be translated into protein. For many eukaryotic introns, splicing occurs in a series of reactions which are catalyzed by the spliceosome, a complex of small nuclear ribonucleoproteins (snRNPs). There exist self-splicing introns, that is, ribozymes that can catalyze their own excision from their parent RNA molecule. The process of transcription, splicing and translation is called gene expression, the central dogma of molecular biology.
The coding region of a gene, also known as the coding sequence (CDS), is the portion of a gene's DNA or RNA that codes for a protein. Studying the length, composition, regulation, splicing, structures, and functions of coding regions compared to non-coding regions over different species and time periods can provide a significant amount of important information regarding gene organization and evolution of prokaryotes and eukaryotes. This can further assist in mapping the human genome and developing gene therapy.

Alternative splicing, or alternative RNA splicing, or differential splicing, is an alternative splicing process during gene expression that allows a single gene to code for multiple proteins. In this process, particular exons of a gene may be included within or excluded from the final, processed messenger RNA (mRNA) produced from that gene. This means the exons are joined in different combinations, leading to different (alternative) mRNA strands. Consequently, the proteins translated from alternatively spliced mRNAs usually contain differences in their amino acid sequence and, often, in their biological functions.

Silent mutations are mutations in DNA that do not have an observable effect on the organism's phenotype. They are a specific type of neutral mutation. The phrase silent mutation is often used interchangeably with the phrase synonymous mutation; however, synonymous mutations are not always silent, nor vice versa. Synonymous mutations can affect transcription, splicing, mRNA transport, and translation, any of which could alter phenotype, rendering the synonymous mutation non-silent. The substrate specificity of the tRNA to the rare codon can affect the timing of translation, and in turn the co-translational folding of the protein. This is reflected in the codon usage bias that is observed in many species. Mutations that cause the altered codon to produce an amino acid with similar functionality are often classified as silent; if the properties of the amino acid are conserved, this mutation does not usually significantly affect protein function.
IKBKAP is a human gene encoding the IKAP protein, which is ubiquitously expressed at varying levels in all tissue types, including brain cells. The IKAP protein is thought to participate as a sub-unit in the assembly of a six-protein putative human holo-Elongator complex, which allows for transcriptional elongation by RNA polymerase II. Further evidence has implicated the IKAP protein as being critical in neuronal development, and directs that decreased expression of IKAP in certain cell types is the molecular basis for the severe, neurodevelopmental disorder familial dysautonomia. Other pathways that have been connected to IKAP protein function in a variety of organisms include tRNA modification, cell motility, and cytosolic stress signalling. Homologs of the IKBKAP gene have been identified in multiple other Eukaryotic model organisms. Notable homologs include Elp1 in yeast, Ikbkap in mice, and D-elp1 in fruit flies. The fruit fly homolog (D-elp1) has RNA-dependent RNA polymerase activity and is involved in RNA interference.
Exon shuffling is a molecular mechanism for the formation of new genes. It is a process through which two or more exons from different genes can be brought together ectopically, or the same exon can be duplicated, to create a new exon-intron structure. There are different mechanisms through which exon shuffling occurs: transposon mediated exon shuffling, crossover during sexual recombination of parental genomes and illegitimate recombination.
In molecular biology, an exonic splicing enhancer (ESE) is a DNA sequence motif consisting of 6 bases within an exon that directs, or enhances, accurate splicing of heterogeneous nuclear RNA (hnRNA) or pre-mRNA into messenger RNA (mRNA).
An exonic splicing silencer (ESS) is a short region of an exon and is a cis-regulatory element. A set of 103 hexanucleotides known as FAS-hex3 has been shown to be abundant in ESS regions. ESSs inhibit or silence splicing of the pre-mRNA and contribute to constitutive and alternate splicing. To elicit the silencing effect, ESSs recruit proteins that will negatively affect the core splicing machinery.

Cystatin-B is a protein that in humans is encoded by the CSTB gene.

60S ribosomal protein L41 is a protein that is specific to humans and is encoded by the RPL41 gene, also known as HG12 and large eukaryotic ribosomal subunit protein eL41. The gene family HGNC is L ribosomal proteins. The protein itself is also described as P62945-RL41_HUMAN on the GeneCards database. This RPL41 gene is located on chromosome 12.
Periannan Senapathy is a molecular biologist, geneticist, author and entrepreneur. He is the founder, president and chief scientific officer at Genome International Corporation, a biotechnology, bioinformatics, and information technology firm based in Madison, Wisconsin, which develops computational genomics applications of next-generation DNA sequencing (NGS) and clinical decision support systems for analyzing patient genome data that aids in diagnosis and treatment of diseases.

Kohlschütter–Tönz syndrome (KTS), also called amelo-cerebro-hypohidrotic syndrome, is a rare inherited syndrome characterized by epilepsy, psychomotor delay or regression, intellectual disability, and yellow teeth caused by amelogenesis imperfecta. It is a type A ectodermal dysplasia.
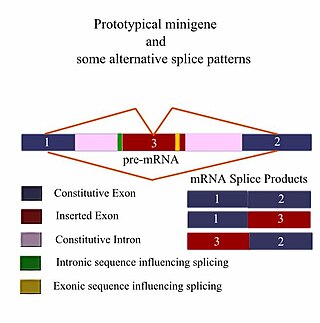
A minigene is a minimal gene fragment that includes an exon and the control regions necessary for the gene to express itself in the same way as a wild type gene fragment. This is a minigene in its most basic sense. More complex minigenes can be constructed containing multiple exons and intron(s). Minigenes provide a valuable tool for researchers evaluating splicing patterns both in vivo and in vitro biochemically assessed experiments. Specifically, minigenes are used as splice reporter vectors and act as a probe to determine which factors are important in splicing outcomes. They can be constructed to test the way both cis-regulatory elements and trans-regulatory elements affect gene expression.
Exitrons are produced through alternative splicing and have characteristics of both introns and exons, but are described as retained introns. Even though they are considered introns, which are typically cut out of pre mRNA sequences, there are significant problems that arise when exitrons are spliced out of these strands, with the most obvious result being altered protein structures and functions. They were first discovered in plants, but have recently been found in metazoan species as well.

Prp8 refers to both the Prp8 protein and Prp8 gene. Prp8's name originates from its involvement in pre-mRNA processing. The Prp8 protein is a large, highly conserved, and unique protein that resides in the catalytic core of the spliceosome and has been found to have a central role in molecular rearrangements that occur there. Prp8 protein is a major central component of the catalytic core in the spliceosome, and the spliceosome is responsible for splicing of precursor mRNA that contains introns and exons. Unexpressed introns are removed by the spliceosome complex in order to create a more concise mRNA transcript. Splicing is just one of many different post-transcriptional modifications that mRNA must undergo before translation. Prp8 has also been hypothesized to be a cofactor in RNA catalysis.
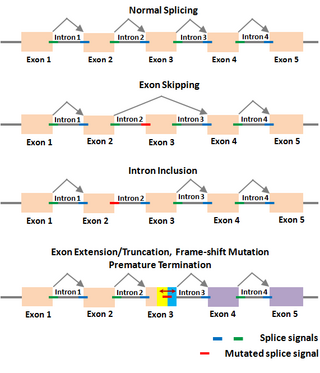
The Shapiro—Senapathy algorithm (S&S) is an algorithm for predicting splice junctions in genes of animals and plants. This algorithm has been used to discover disease-causing splice site mutations and cryptic splice sites.
The split gene theory is a theory of the origin of introns, long non-coding sequences in eukaryotic genes between the exons. The theory holds that the randomness of primordial DNA sequences would only permit small (< 600bp) open reading frames (ORFs), and that important intron structures and regulatory sequences are derived from stop codons. In this introns-first framework, the spliceosomal machinery and the nucleus evolved due to the necessity to join these ORFs into larger proteins, and that intronless bacterial genes are less ancestral than the split eukaryotic genes. The theory originated with Periannan Senapathy.











