
A steam engine is a heat engine that performs mechanical work using steam as its working fluid. The steam engine uses the force produced by steam pressure to push a piston back and forth inside a cylinder. This pushing force can be transformed, by a connecting rod and crank, into rotational force for work. The term "steam engine" is most commonly applied to reciprocating engines as just described, although some authorities have also referred to the steam turbine and devices such as Hero's aeolipile as "steam engines". The essential feature of steam engines is that they are external combustion engines, where the working fluid is separated from the combustion products. The ideal thermodynamic cycle used to analyze this process is called the Rankine cycle. In general usage, the term steam engine can refer to either complete steam plants, such as railway steam locomotives and portable engines, or may refer to the piston or turbine machinery alone, as in the beam engine and stationary steam engine.

Boiling or ebullition is the rapid phase transition from liquid to gas or vapor; the reverse of boiling is condensation. Boiling occurs when a liquid is heated to its boiling point, so that the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere. Boiling and evaporation are the two main forms of liquid vapourization.

A boiler is a closed vessel in which fluid is heated. The fluid does not necessarily boil. The heated or vaporized fluid exits the boiler for use in various processes or heating applications, including water heating, central heating, boiler-based power generation, cooking, and sanitation.

A combined cycle power plant is an assembly of heat engines that work in tandem from the same source of heat, converting it into mechanical energy. On land, when used to make electricity the most common type is called a combined cycle gas turbine (CCGT) plant, which is a kind of gas-fired power plant. The same principle is also used for marine propulsion, where it is called a combined gas and steam (COGAS) plant. Combining two or more thermodynamic cycles improves overall efficiency, which reduces fuel costs.

The Rankine cycle is an idealized thermodynamic cycle describing the process by which certain heat engines, such as steam turbines or reciprocating steam engines, allow mechanical work to be extracted from a fluid as it moves between a heat source and heat sink. The Rankine cycle is named after William John Macquorn Rankine, a Scottish polymath professor at Glasgow University.
For fluid power, a working fluid is a gas or liquid that primarily transfers force, motion, or mechanical energy. In hydraulics, water or hydraulic fluid transfers force between hydraulic components such as hydraulic pumps, hydraulic cylinders, and hydraulic motors that are assembled into hydraulic machinery, hydraulic drive systems, etc. In pneumatics, the working fluid is air or another gas which transfers force between pneumatic components such as compressors, vacuum pumps, pneumatic cylinders, and pneumatic motors. In pneumatic systems, the working gas also stores energy because it is compressible.

Superheated steam is steam at a temperature higher than its vaporization point at the absolute pressure where the temperature is measured.

A steam explosion is an explosion caused by violent boiling or flashing of water or ice into steam, occurring when water or ice is either superheated, rapidly heated by fine hot debris produced within it, or heated by the interaction of molten metals. Steam explosions are instances of explosive boiling. Pressure vessels, such as pressurized water (nuclear) reactors, that operate above atmospheric pressure can also provide the conditions for a steam explosion. The water changes from a solid or liquid to a gas with extreme speed, increasing dramatically in volume. A steam explosion sprays steam and boiling-hot water and the hot medium that heated it in all directions, creating a danger of scalding and burning.
A superheater is a device used to convert saturated steam or wet steam into superheated steam or dry steam. Superheated steam is used in steam turbines for electricity generation, in some steam engines, and in processes such as steam reforming. There are three types of superheaters: radiant, convection, and separately fired. A superheater can vary in size from a few tens of feet to several hundred feet.

A thermal power station is a type of power station in which heat energy is converted to electrical energy. In a steam-generating cycle heat is used to boil water in a large pressure vessel to produce high-pressure steam, which drives a steam turbine connected to an electrical generator. The low-pressure exhaust from the turbine enters a steam condenser where it is cooled to produce hot condensate which is recycled to the heating process to generate more high pressure steam. This is known as a Rankine cycle.

The steam-electric power station is a power station in which the electric generator is steam driven. Water is heated, turns into steam and spins a steam turbine which drives an electrical generator. After it passes through the turbine, the steam is condensed in a condenser. The greatest variation in the design of steam-electric power plants is due to the different fuel sources.
Economizers, or economisers (UK), are mechanical devices intended to reduce energy consumption, or to perform useful function such as preheating a fluid. The term economizer is used for other purposes as well. Boiler, power plant, heating, refrigeration, ventilating, and air conditioning (HVAC) uses are discussed in this article. In simple terms, an economizer is a heat exchanger.

Vapour-compression refrigeration or vapor-compression refrigeration system (VCRS), in which the refrigerant undergoes phase changes, is one of the many refrigeration cycles and is the most widely used method for air conditioning of buildings and automobiles. It is also used in domestic and commercial refrigerators, large-scale warehouses for chilled or frozen storage of foods and meats, refrigerated trucks and railroad cars, and a host of other commercial and industrial services. Oil refineries, petrochemical and chemical processing plants, and natural gas processing plants are among the many types of industrial plants that often utilize large vapor-compression refrigeration systems. Cascade refrigeration systems may also be implemented using two compressors.

An evaporator is a type of heat exchanger device that facilitates evaporation by utilizing conductive and convective heat transfer, which provides the necessary thermal energy for phase transition from liquid to vapor. Within evaporators, a circulating liquid is exposed to an atmospheric or reduced pressure environment, causing it to boil at a lower temperature compared to normal atmospheric boiling.

In thermodynamics, vapor quality is the mass fraction in a saturated mixture that is vapor; in other words, saturated vapor has a "quality" of 100%, and saturated liquid has a "quality" of 0%. Vapor quality is an intensive property which can be used in conjunction with other independent intensive properties to specify the thermodynamic state of the working fluid of a thermodynamic system. It has no meaning for substances which are not saturated mixtures . Vapor quality is an important quantity during the adiabatic expansion step in various thermodynamic cycles. Working fluids can be classified by using the appearance of droplets in the vapor during the expansion step.

A boiler or steam generator is a device used to create steam by applying heat energy to water. Although the definitions are somewhat flexible, it can be said that older steam generators were commonly termed boilers and worked at low to medium pressure but, at pressures above this, it is more usual to speak of a steam generator.

In thermal engineering, the organic Rankine cycle (ORC) is a type of thermodynamic cycle. It is a variation of the Rankine cycle named for its use of an organic, high-molecular-mass fluid whose vaporization temperature is lower than that of water. The fluid allows heat recovery from lower-temperature sources such as biomass combustion, industrial waste heat, geothermal heat, solar ponds etc. The low-temperature heat is converted into useful work, that can itself be converted into electricity.
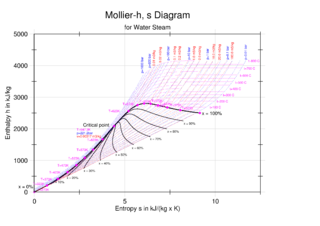
An enthalpy–entropy chart, also known as the H–S chart or Mollier diagram, plots the total heat against entropy, describing the enthalpy of a thermodynamic system. A typical chart covers a pressure range of 0.01–1000 bar, and temperatures up to 800 degrees Celsius. It shows enthalpy in terms of internal energy , pressure and volume using the relationship .

The Hygroscopic cycle is a thermodynamic cycle converting thermal energy into mechanical power by the means of a steam turbine. It is similar to the Rankine cycle using water as the motive fluid but with the novelty of introducing salts and their hygroscopic properties for the condensation. The salts are desorbed in the boiler or steam generator, where clean steam is released and superheated in order to be expanded and generate power through the steam turbine. Boiler blowdown with the concentrated hygroscopic compounds is used thermally to pre-heat the steam turbine condensate, and as reflux in the steam-absorber.
Heat engines, refrigeration cycles and heat pumps usually involve a fluid to and from which heat is transferred while undergoing a thermodynamic cycle. This fluid is called the working fluid. Refrigeration and heat pump technologies often refer to working fluids as refrigerants. Most thermodynamic cycles make use of the latent heat of the working fluid. In case of other cycles the working fluid remains in gaseous phase while undergoing all the processes of the cycle. When it comes to heat engines, working fluid generally undergoes a combustion process as well, for example in internal combustion engines or gas turbines. There are also technologies in heat pump and refrigeration, where working fluid does not change phase, such as reverse Brayton or Stirling cycle.






















