Related Research Articles

The combined oral contraceptive pill (COCP), often referred to as the birth control pill or colloquially as "the pill", is a type of birth control that is designed to be taken orally by women. The pill contains two important hormones: a progestin and estrogen. When taken correctly, it alters the menstrual cycle to eliminate ovulation and prevent pregnancy.

Percy Lavon Julian was an American research chemist and a pioneer in the chemical synthesis of medicinal drugs from plants. He was the first to synthesize the natural product physostigmine and was a pioneer in the industrial large-scale chemical synthesis of the human hormones progesterone and testosterone from plant sterols such as stigmasterol and sitosterol. His work laid the foundation for the steroid drug industry's production of cortisone, other corticosteroids, and birth control pills.

Carl Djerassi was an Austrian-born Bulgarian-American pharmaceutical chemist, novelist, playwright and co-founder of Djerassi Resident Artists Program with Diane Wood Middlebrook. He is best known for his contribution to the development of oral contraceptive pills, nicknamed the "father of the pill".
Russell Earl Marker was an American chemist who invented the octane rating system when he was working at the Ethyl Corporation. Later in his career, he went on to found a steroid industry in Mexico when he successfully made semisynthetic progesterone from chemical constituents found in Mexican yams in a process known as Marker degradation. This eventually led to the development at Syntex of the combined oral contraceptive pill and synthetic cortisone – and to the development of the Mexican barbasco trade.

Luis Ernesto Miramontes Cárdenas was a Mexican chemist known as the co-inventor of the progestin norethisterone used in one of the first three oral contraceptives.
Franz Sondheimer FRS was a German-born British professor of chemistry. In 1960, he was awarded the Israel Prize for his contributions to science.

George Rosenkranz was a pioneering Mexican scientist in the field of steroid chemistry, who used native Mexican plant sources as raw materials. He was born in Hungary, studied in Switzerland and emigrated to the Americas to escape the Nazis, eventually settling in Mexico.

Alejandro Zaffaroni was a Uruguayan serial entrepreneur who was responsible for founding several biotechnology companies in Silicon Valley. Products that he was involved in developing include the birth control pill, the nicotine patch, corticosteroids, and the DNA microarray.

Norethisterone, also known as norethindrone and sold under many brand names, is a progestin medication used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication is available in both low-dose and high-dose formulations and both alone and in combination with an estrogen. It is used by mouth or, as norethisterone enanthate, by injection into muscle.

Lynestrenol, sold under the brand names Exluton and Ministat among others, is a progestin medication which is used in birth control pills and in the treatment of gynecological disorders. The medication is available both alone and in combination with an estrogen. It is taken by mouth.

Ethisterone, also known as ethinyltestosterone, pregneninolone, and anhydrohydroxyprogesterone and formerly sold under the brand names Proluton C and Pranone among others, is a progestin medication which was used in the treatment of gynecological disorders but is now no longer available. It was used alone and was not formulated in combination with an estrogen. The medication is taken by mouth.

Diosgenin, a phytosteroid sapogenin, is the product of hydrolysis by acids, strong bases, or enzymes of saponins, extracted from the tubers of Dioscorea wild yam species, such as the Kokoro. The sugar-free (aglycone) product of such hydrolysis, diosgenin is used for the commercial synthesis of cortisone, pregnenolone, progesterone, and other steroid products.
Frank Benjamin Colton, American chemist who first synthesized noretynodrel, the progestin used in Enovid, the first oral contraceptive, at G. D. Searle & Company in Skokie, Illinois in 1952.
The Marker degradation is a three-step synthetic route in steroid chemistry developed by American chemist Russell Earl Marker in 1938–40. It is used for the production of cortisone and mammalian sex hormones from plant steroids, and established Mexico as a world center for steroid production in the years immediately after World War II. The discovery of the Marker degradation allowed the production of substantial quantities of steroid hormones for the first time, and was fundamental in the development of the contraceptive pill and corticosteroid anti-inflammatory drugs. In 1999, the American Chemical Society and the Sociedad Química de México named the route as an International Historic Chemical Landmark.

Noretynodrel, or norethynodrel, sold under the brand name Enovid among others, is a progestin medication which was previously used in birth control pills and in the treatment of gynecological disorders but is now no longer marketed. It was available both alone and in combination with an estrogen. The medication is taken by mouth.

Dioscorea composita, or barbasco, is a species of yam in the genus Dioscorea, native to Mexico. It is notable for its role in the production of diosgenin, which is a precursor for the synthesis of hormones such as progesterone. Russell Marker developed the extraction and manufacture of hormones from D. mexicana at Syntex, starting the trade of D. composita in Mexico. Marker also discovered that the composita variety had a much higher content of diosgenin than the mexicana variety, and therefore it came to replace the latter in the production of synthetic hormones.

The Mexican barbasco trade was the trade of the diosgenin-rich yam species Dioscorea mexicana, Dioscorea floribunda and Dioscorea composita which emerged in Mexico in the 1950s as part of the Mexican steroid industry. The trade consisted in Mexican campesinos harvesting the root in the jungle, selling it to middlemen who brought it to processing plants where the root was fermented and the diosgenin extracted and sold to pharmaceutical companies such as Syntex who used it to produce synthetic hormones.
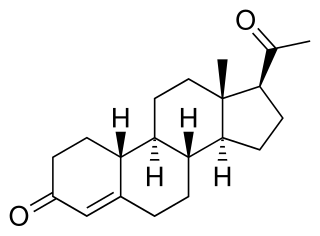
19-Norprogesterone, also known as 19-norpregn-4-ene-3,20-dione, is a steroidal progestin and close analogue of the sex hormone progesterone, lacking only the C19 methyl group of that molecule. It was first synthesized in 1944 in the form of a mixture that also included unnatural stereoisomers of progesterone, and this mixture was found to be at least equivalent to progesterone in terms of progestogenic activity. Subsequent investigations revealed that 17-isoprogesterone and 14-iso-17-isoprogesterone are devoid of progestogenic activity. 19-Norprogesterone was resynthesized in 1951 with an improved method, and was confirmed to be the component of the mixture synthesized in 1944 that was responsible for its progestogenic activity. In 1953, a paper was published showing that 19-norprogesterone possessed 4- to 8-fold the activity of progesterone in the Clauberg assay in rabbits, and at the time of this discovery, 19-norprogesterone was the most potent progestogen known.
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne is added to a carbonyl group to form an α-alkynyl alcohol.
Progestogen-only injectable contraceptives (POICs) are a form of hormonal contraception and progestogen-only contraception that are administered by injection and providing long-lasting birth control. As opposed to combined injectable contraceptives, they contain only a progestogen without an estrogen, and include two progestin preparations:
References
- ↑ Gereffi, Gary (1983). The Pharmaceutical Industry and Dependency in the Third World (2017 reprint ed.). Princeton University Press: Princeton. p. 83. ISBN 9781400886227 . Retrieved 11 January 2021.
- ↑ Laveaga, Gabriela Soto (2009). Jungle Laboratories: Mexican Peasants, National Projects, and the Making of the Pill. Durham: Duke University Press. p. 3. ISBN 9780822391968 . Retrieved 11 January 2021.
- ↑ "The 'Marker Degradation' & Creation of the Mexican Steroid Hormone Industry 1938–1945" (PDF). ACS.org. 1999.
- ↑ McFadden, Robert D. (2019-06-23). "George Rosenkranz, 102, a Developer of the Birth Control Pill, Is Dead". The New York Times. ISSN 0362-4331 . Retrieved 2023-02-03.
- ↑ Gereffi, Gary (1983). The Pharmaceutical Industry and Dependency in the Third World (2017 reprint ed.). Princeton University Press: Princeton. p. 110. ISBN 9781400886227 . Retrieved 11 January 2021.
- ↑ Freudenheim, Milt (3 May 1994). "Roche Set To Acquire Syntex". New York Times.
- ↑ Oremus, Will (September 2, 2008). "Roche confirms closing of Palo Alto complex". San Jose Mercury News.
- ↑ Sheyner, Gennady (November 21, 2011). "VMware unveils plan for massive campus expansion". Palo Alto Online. Retrieved 3 September 2021.
- ↑ Rosenkranz had fled Nazi Germany to avoid the eventual Holocaust; while in Cuba en route to South America, the Japanese attack on Pearl Harbor stranded him there. He was able to work there for the duration of the war.
- ↑ Marshall, Eliot (1983-06-10). "The murky world of toxicity testing" (PDF). Science. 220 (4602): 1130–1132. Bibcode:1983Sci...220.1130M. doi:10.1126/science.6857237. PMID 6857237 . Retrieved 2012-07-27.
- ↑ "The Scandal in Chemical Testing". The New York Times. 1983-05-16. Retrieved 2012-07-27.
The problem was discovered only by accident, when a Government official looking for something else pulled out a file of IBT data by mistake.
- ↑ Merrell, Paul (Winter 1981). "The Industrial Bio-Test Caper" (PDF). NCAP News. 2 (3): 2–4. Archived from the original (PDF) on 2014-02-23. Retrieved 2012-08-05.
- ↑ Foster, Douglas; Mark Dowie; Steve Hubbell; Irene Moosen; Peter Waldman; Center for Investigative Reporting (June 1982). "Poisoned Research". Mother Jones. 7 (5): 38–40, 42–43, 45–48. Retrieved 2012-07-28.