Anti-thymocyte globulin (ATG) is an infusion of horse or rabbit-derived antibodies against human T cells and their precursors (thymocytes), which is used in the prevention and treatment of acute rejection in organ transplantation and therapy of aplastic anemia due to bone marrow insufficiency.
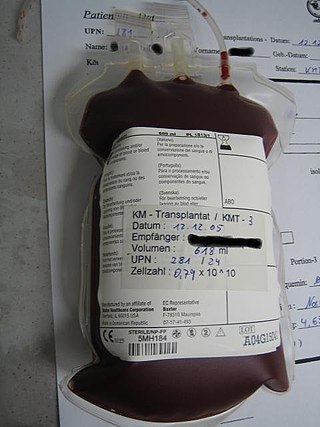
Hematopoietic stem-cell transplantation (HSCT) is the transplantation of multipotent hematopoietic stem cells, usually derived from bone marrow, peripheral blood, or umbilical cord blood in order to replicate inside of a patient and to produce additional normal blood cells. It may be autologous, allogeneic or syngeneic.

Graft-versus-host disease (GvHD) is a syndrome, characterized by inflammation in different organs. GvHD is commonly associated with bone marrow transplants and stem cell transplants.

Thymidine kinase is an enzyme, a phosphotransferase : 2'-deoxythymidine kinase, ATP-thymidine 5'-phosphotransferase, EC 2.7.1.21. It can be found in most living cells. It is present in two forms in mammalian cells, TK1 and TK2. Certain viruses also have genetic information for expression of viral thymidine kinases. Thymidine kinase catalyzes the reaction:

Alemtuzumab, sold under the brand names Campath and Lemtrada among others, is a medication used to treat chronic lymphocytic leukemia and multiple sclerosis. In chronic lymphocytic leukemia, it has been used as both a first line and second line treatment. It is given by injection into a vein.

X-linked severe combined immunodeficiency (X-SCID) is an immunodeficiency disorder in which the body produces very few T cells and NK cells.

Herpes simplex virus1 and 2, also known by their taxonomic names Human alphaherpesvirus 1 and Human alphaherpesvirus 2, are two members of the human Herpesviridae family, a set of viruses that produce viral infections in the majority of humans. Both HSV-1 and HSV-2 are very common and contagious. They can be spread when an infected person begins shedding the virus.
Transfusion-associated graft-versus-host disease (TA-GvHD) is a rare complication of blood transfusion, in which the immunologically competent donor T lymphocytes mount an immune response against the recipient's lymphoid tissue. These donor lymphocytes engraft, recognize recipient cells as foreign and mount an immune response against recipient tissues. Donor lymphocytes are usually identified as foreign and destroyed by the recipient's immune system. However, in situations where the recipient is severely immunocompromised, or when the donor and recipient HLA type is similar, the recipient's immune system is not able to destroy the donor lymphocytes. This can result in transfusion associated graft-versus-host disease. This is in contrast with organ/tissue transplant associated GvHD, where matching HLA reduces the incident of the complication.
Juvenile myelomonocytic leukemia (JMML) is a rare form of chronic leukemia that affects children, commonly those aged four and younger. The name JMML now encompasses all diagnoses formerly referred to as juvenile chronic myeloid leukemia (JCML), chronic myelomonocytic leukemia of infancy, and infantile monosomy 7 syndrome. The average age of patients at diagnosis is two (2) years old. The World Health Organization has included JMML as a subcategory of myelodysplastic and myeloproliferative disorders.
Donor lymphocyte infusion (DLI) or buffy coat infusion is a form of adoptive immunotherapy used after hematopoietic stem cell transplantation.

Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare hematologic malignancy. It was initially regarded as a form of lymphocyte-derived cutaneous lymphoma and alternatively named CD4+CD56+ hematodermic tumor, blastic NK cell lymphoma, and agranular CD4+ NK cell leukemia. Later, however, the disease was determined to be a malignancy of plasmacytoid dendritic cells rather than lymphocytes and therefore termed blastic plasmacytoid dendritic cell neoplasm. In 2016, the World Health Organization designated BPDCN to be in its own separate category within the myeloid class of neoplasms. It is estimated that BPDCN constitutes 0.44% of all hematological malignancies.
Herpes simplex research includes all medical research that attempts to prevent, treat, or cure herpes, as well as fundamental research about the nature of herpes. Examples of particular herpes research include drug development, vaccines and genome editing. HSV-1 and HSV-2 are commonly thought of as oral and genital herpes respectively, but other members in the herpes family include chickenpox (varicella/zoster), cytomegalovirus, and Epstein-Barr virus. There are many more virus members that infect animals other than humans, some of which cause disease in companion animals or have economic impacts in the agriculture industry.

Herpetic simplex keratitis is a form of keratitis caused by recurrent herpes simplex virus (HSV) infection in the cornea.

Pritelivir is a direct-acting antiviral drug in development for the treatment of herpes simplex virus infections (HSV). This is particularly important in immune compromised patients. Pritelivir is currently in Phase III clinical development by the German biopharmaceutical company AiCuris Anti-infective Cures AG.
Graft-versus-tumor effect (GvT) appears after allogeneic hematopoietic stem cell transplantation (HSCT). The graft contains donor T cells that can be beneficial for the recipient by eliminating residual malignant cells. GvT might develop after recognizing tumor-specific or recipient-specific alloantigens. It could lead to remission or immune control of hematologic malignancies. This effect applies in myeloma and lymphoid leukemias, lymphoma, multiple myeloma and possibly breast cancer. It is closely linked with graft-versus-host disease (GvHD), as the underlying principle of alloimmunity is the same. CD4+CD25+ regulatory T cells (Treg) can be used to suppress GvHD without loss of beneficial GvT effect. The biology of GvT response is still not fully understood but it is probable that the reaction with polymorphic minor histocompatibility antigens expressed either specifically on hematopoietic cells or more widely on a number of tissue cells or tumor-associated antigens is involved. This response is mediated largely by cytotoxic T lymphocytes (CTL) but it can be employed by natural killers as separate effectors, particularly in T-cell-depleted HLA-haploidentical HSCT.
Microtransplantation (MST) is an advanced technology to treat malignant hematological diseases and tumors by infusing patients with granulocyte colony-stimulating factor (G-CSF) mobilized human leukocyte antigen (HLA)-mismatched allogeneic peripheral blood stem cells following a reduced-intensity chemotherapy or targeted therapy. The term "microtransplantation" comes from its mechanism of reaching donor cell microchimerism.
Guo Mei is a hematologist and associate director of 307th Hospital of Chinese People’s Liberation Army and deputy director of Radiation Research Institute.
T-cell depletion (TCD) is the process of T cell removal or reduction, which alters the immune system and its responses. Depletion can occur naturally or be induced for treatment purposes. TCD can reduce the risk of graft-versus-host disease (GVHD), which is a common issue in transplants. The idea that TCD of the allograft can eliminate GVHD was first introduced in 1958. In humans the first TCD was performed in severe combined immunodeficiency patients.

Shimon Slavin is an Israeli professor of medicine. Slavin pioneered the use of immunotherapy mediated by allogeneic donor lymphocytes and innovative methods for stem cell transplantation for the cure of hematological malignancies and solid tumors, and using hematopoietic stem cells for induction of transplantation tolerance to bone marrow and donor allografts.
Maria Grazia Roncarolo is an Italian pediatrician who is currently George D. Smith Professor in Stem Cell and Regenerative Medicine and Professor of Medicine at Stanford University. She is also the Director of the Stanford Institute of Stem Cell Biology and Regenerative Medicine along with Irving Weissman and Michael Longaker and the Director for Center for Definitive and Curative Medicine at Stanford.









