
Aromatic compounds usually refers to organic compounds "with a chemistry typified by benzene" and "cyclically conjugated " The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's Rule. Aromatic compounds have the following general properties:

A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles.

Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical study.

Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.

In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds.

Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.

Phosphorine is a heavier element analog of pyridine, containing a phosphorus atom instead of an aza- moiety. It is also called phosphabenzene and belongs to the phosphaalkene class. It is a colorless liquid that is mainly of interest in research.
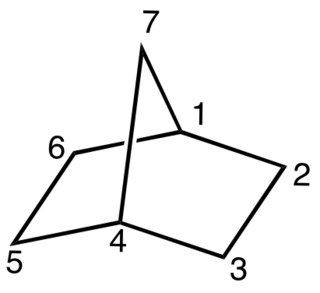
A bicyclic molecule is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic, or heterocyclic, like DABCO. Moreover, the two rings can both be aliphatic, or can be aromatic, or a combination of aliphatic and aromatic.

Borabenzene is a hypothetical organoboron compound with the formula C5H5B. Unlike the related but highly stable benzene molecule, borabenzene would be electron-deficient. Related derivatives are the boratabenzene anions, including the parent [C5H5BH]−.

Isoquinoline is an individual chemical specimen - a heterocyclic aromatic organic compound - as well as the name of a family of many thousands of natural plant alkaloids, any one of which might be referred to as "an isoquinoline". It is a structural isomer of quinoline. Isoquinoline and quinoline are benzopyridines, which are composed of a benzene ring fused to a pyridine ring. In a broader sense, the term isoquinoline is used to make reference to isoquinoline derivatives. 1-Benzylisoquinoline is the structural backbone in many naturally occurring alkaloids such as papaverine. The isoquinoline ring in these natural compound derives from the aromatic amino acid tyrosine.

Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak base; its conjugate acid has a pKa of 0.8, compared to 7 for imidazole.
Thiazole, or 1,3-thiazole, is a 5-membered heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1).
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.
Pyrylium is a cation with formula C5H5O+, consisting of a six-membered ring of five carbon atoms, each with one hydrogen atom, and one positively charged oxygen atom. The bonds in the ring are conjugated as in benzene, giving it an aromatic character. In particular, because of the positive charge, the oxygen atom is trivalent. Pyrilium is a mono-cyclic and heterocyclic compound, one of the oxonium ions.
Stannabenzene (C5H6Sn) is the parent representative of a group of organotin compounds that are related to benzene with a carbon atom replaced by a tin atom. Stannabenzene itself has been studied by computational chemistry, but has not been isolated.
Arsabenzene (IUPAC name: arsinine) is an organoarsenic heterocyclic compound with the chemical formula C5H5As. It belongs to a group of compounds called heteroarenes that have the general formula C5H5E (E= N, P, As, Sb, Bi).
Thiopyrylium is a cation with the chemical formula C5H5S+. It is analogous to the pyrylium cation with the oxygen atom replaced by a sulfur atom.

Selenopyrylium is an aromatic heterocyclic compound consisting of a six-membered ring with five carbon atoms and a positively charged selenium atom.

Stibinin, also known as stibabenzene, is an organic chemical compound. Stibinin has the chemical formula C5H5Sb. The molecule, stibinin, is a derivative of benzene, with one of the carbon atoms in the 6-membered ring replaced by an antimony (Sb) atom. Stibinin is a molecule that is considered to be an organoantimony compound due to it containing carbon, hydrogen, and antimony atoms.
















