Related Research Articles
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during reactions with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds.
The following outline is provided as an overview of and topical guide to chemistry:

A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.

Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical study.

A polymer is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals.
Physical science is a branch of natural science that studies non-living systems, in contrast to life science. It in turn has many branches, each referred to as a "physical science", together is called the "physical sciences".
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions to physical and chemical properties of molecules, materials, and solutions at the atomic level. These calculations include systematically applied approximations intended to make calculations computationally feasible while still capturing as much information about important contributions to the computed wave functions as well as to observable properties such as structures, spectra, and thermodynamic properties. Quantum chemistry is also concerned with the computation of quantum effects on molecular dynamics and chemical kinetics.

Roald Hoffmann is a Polish-American theoretical chemist who won the 1981 Nobel Prize in Chemistry. He has also published plays and poetry. He is the Frank H. T. Rhodes Professor of Humane Letters Emeritus at Cornell University.

Hideki Shirakawa is a Japanese chemist, engineer, and Professor Emeritus at the University of Tsukuba and Zhejiang University. He is best known for his discovery of conductive polymers. He was co-recipient of the 2000 Nobel Prize in Chemistry jointly with Alan MacDiarmid and Alan Heeger.
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects.
Carbon is a primary component of all known life on Earth, and represents approximately 45–50% of all dry biomass. Carbon compounds occur naturally in great abundance on Earth. Complex biological molecules consist of carbon atoms bonded with other elements, especially oxygen and hydrogen and frequently also nitrogen, phosphorus, and sulfur.
The Willard Gibbs Award, presented by the Chicago Section of the American Chemical Society, was established in 1910 by William A. Converse (1862–1940), a former Chairman and Secretary of the Chicago Section of the society and named for Professor Josiah Willard Gibbs (1839–1903) of Yale University. Gibbs, whose formulation of the Phase Rule founded a new science, is considered by many to be the only American-born scientist whose discoveries are as fundamental in nature as those of Newton and Galileo.

This timeline of chemistry lists important works, discoveries, ideas, inventions, and experiments that significantly changed humanity's understanding of the modern science known as chemistry, defined as the scientific study of the composition of matter and of its interactions.
Physical organic chemistry, a term coined by Louis Hammett in 1940, refers to a discipline of organic chemistry that focuses on the relationship between chemical structures and reactivity, in particular, applying experimental tools of physical chemistry to the study of organic molecules. Specific focal points of study include the rates of organic reactions, the relative chemical stabilities of the starting materials, reactive intermediates, transition states, and products of chemical reactions, and non-covalent aspects of solvation and molecular interactions that influence chemical reactivity. Such studies provide theoretical and practical frameworks to understand how changes in structure in solution or solid-state contexts impact reaction mechanism and rate for each organic reaction of interest.
Herbert Sander Gutowsky was an American chemist who was a professor of chemistry at the University of Illinois Urbana-Champaign. Gutowsky was the first to apply nuclear magnetic resonance (NMR) methods to the field of chemistry. He used nuclear magnetic resonance spectroscopy to determine the structure of molecules. His pioneering work developed experimental control of NMR as a scientific instrument, connected experimental observations with theoretical models, and made NMR one of the most effective analytical tools for analysis of molecular structure and dynamics in liquids, solids, and gases, used in chemical and medical research, His work was relevant to the solving of problems in chemistry, biochemistry, and materials science, and has influenced many of the subfields of more recent NMR spectroscopy.

James Carl Stevens, a chemist, was the first Distinguished Fellow, at the Dow Chemical Company, retiring in January 2015. His area of expertise is organometallic chemistry and his primary field of research is in the area of polyolefin catalysis, particularly in the area of polyethylene, polypropylene, ethylene/styrene copolymers, and the combinatorial discovery of organometallic single-site catalysts. Stevens major contributions have come in the discovery and commercial implementation of single-site polyolefin catalysts. He invented and led the commercialization of constrained geometry catalyst for the polymerization of olefins. These have been commercialized by Dow as a number of polymers, elastomers and plostomers.

Periodic Videos is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics in chemistry and related fields. They are published on YouTube and produced by Brady Haran, a former BBC video journalist, mainly featuring Sir Martyn Poliakoff, Peter Licence, Stephen Liddle, Debbie Kays, Neil Barnes, Sam Tang, and other scientists at the University of Nottingham.
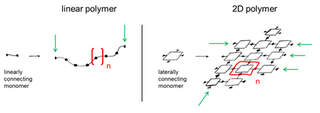
A two-dimensional polymer (2DP) is a sheet-like monomolecular macromolecule consisting of laterally connected repeat units with end groups along all edges. This recent definition of 2DP is based on Hermann Staudinger's polymer concept from the 1920s. According to this, covalent long chain molecules ("Makromoleküle") do exist and are composed of a sequence of linearly connected repeat units and end groups at both termini.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.

Roeland J. M. Nolte was a Dutch chemist, known for his work in the fields of organic chemistry, biochemistry, polymer chemistry, and supramolecular chemistry. He was an emeritus Royal Netherlands Academy of Arts and Sciences professor and an emeritus professor of organic chemistry at Radboud University in Nijmegen, The Netherlands. Until his death, he held a special chair, i.e. professor of molecular nanotechnology, at Radboud University. Nolte was considered to be one of the pioneers of the field of supramolecular chemistry, which encompasses the design and synthesis of new chemical structures from low molecular weight compounds and biopolymers using non-covalent interactions. He published many studies on supramolecular assembly and biomimetic catalysts, which find applications in the field of nanomaterials and medicine.
References
- ↑ http://www.learner.org/catalog/producers/wccreators.html "Series Demonstrator, Donald Showalter" section under "About the Host, Producers, and Advisors"
- ↑ http://www.learner.org/resources/series61.html "It includes physics and Earth science components, and is also valuable for teachers seeking to review the subject matter." - at the last sentence of the 4th section of text (biggest paragraph) in the "Overview"
- ↑ http://www.learner.org/resources/series61.html "Produced by the University of Maryland and the Educational Film Center. 1990." - at the 6th section of text in the "Overview"
- ↑ http://www.learner.org/resources/series61.html "This series supports nationally recognized science standards — NSTA and NCSESA." - at the 5th section of text in the "Overview"
- ↑ "Resource: The World of Chemistry". www.learner.org:80. Archived from the original on 30 April 2019. Retrieved 13 January 2022.
- ↑ http://www.learner.org/catalog/awards/wccomments.html#Awards the "awards" section at the bottom of the page - last section