Ranbaxy Laboratories Limited was an Indian multinational pharmaceutical company that was incorporated in India in 1961 and remained an entity until 2014. The company went public in 1973. Ownership of Ranbaxy changed twice over the course of its history.
Sanofi S.A. is a French multinational pharmaceutical and healthcare company headquartered in Paris, France. The corporation was established in 1973 and merged with Synthélabo in 1999 to form Sanofi-Synthélabo. In 2004, Sanofi-Synthélabo merged with Aventis and renamed to Sanofi-Aventis, which were each the product of several previous mergers. It changed its name back to Sanofi in May 2011. The company is a component of the Euro Stoxx 50 stock market index. In 2023, the company’s seat in Forbes Global 2000 was 89.

Angiotensin II receptor blockers (ARBs), formally angiotensin II receptor type 1 (AT1) antagonists, also known as angiotensin receptor blockers, angiotensin II receptor antagonists, or AT1 receptor antagonists, are a group of pharmaceuticals that bind to and inhibit the angiotensin II receptor type 1 (AT1) and thereby block the arteriolar contraction and sodium retention effects of renin–angiotensin system.
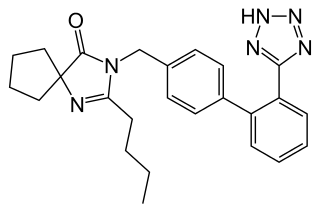
Irbesartan, sold under the brand name Avapro among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease. It is a reasonable initial treatment for high blood pressure. It is taken by mouth. Versions are available as the combination irbesartan/hydrochlorothiazide.

Ranitidine, previously sold under the brand name Zantac among others, is a medication used to decrease stomach acid production. It was commonly used in treatment of peptic ulcer disease, gastroesophageal reflux disease, and Zollinger–Ellison syndrome. It can be given by mouth, injection into a muscle, or injection into a vein.
Dr. Reddy's Laboratories is an Indian multinational pharmaceutical company based in Hyderabad. The company was founded by Kallam Anji Reddy, who previously worked in the mentor institute Indian Drugs and Pharmaceuticals Limited. Dr. Reddy manufactures and markets a wide range of pharmaceuticals in India and overseas. The company produces over 190 medications, 60 active pharmaceutical ingredients (APIs) for drug manufacture, diagnostic kits, critical care, and biotechnology.

Losartan, sold under the brand name Cozaar among others, is a medication used to treat high blood pressure (hypertension). It is in the angiotensin receptor blocker (ARB) family of medication, and is considered protective of the kidneys. Besides hypertension, it is also used in diabetic kidney disease, heart failure, and left ventricular enlargement. It comes as a tablet that is taken by mouth. It may be used alone or in addition to other blood pressure medication. Up to six weeks may be required for the full effects to occur.
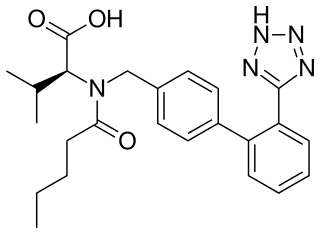
Valsartan, sold under the brand name Diovan among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease. It belongs to a class of medications referred to as angiotensin II receptor blockers (ARBs). It is a reasonable initial treatment for high blood pressure. It is taken by mouth.
Mylan N.V. was a global generic and specialty pharmaceuticals company. In November 2020, Mylan merged with Upjohn, Pfizer's off-patent medicine division, to form Viatris. Previously, the company was domiciled in the Netherlands, with principal executive offices in Hatfield, Hertfordshire, UK and a "Global Center" in Canonsburg, Pennsylvania, US.

Cefpodoxime is an oral, third-generation cephalosporin antibiotic available in various generic preparations. It is active against both Gram-positive and Gram-negative organisms with notable exceptions including Pseudomonas aeruginosa, Enterococcus, and Bacteroides fragilis. It is typically used to treat acute otitis media, pharyngitis, sinusitis, and gonorrhea. It also finds use as oral continuation therapy when intravenous cephalosporins are no longer necessary for continued treatment.

Teva Pharmaceutical Industries Ltd. is an Israeli multinational pharmaceutical company. Teva specializes primarily in generic drugs, but other business interests include branded-drugs, active pharmaceutical ingredients (API's) and, to a lesser extent, contract manufacturing services and an out-licensing platform.

N-Nitrosodimethylamine (NDMA), also known as dimethylnitrosamine (DMN), is an organic compound with the formula (CH3)2NNO. It is one of the simplest members of a large class of nitrosamines. It is a volatile yellow oil. NDMA has attracted wide attention as being highly hepatotoxic and a known carcinogen in laboratory animals.

N-Nitrosodiethylamine (NDEA) is an organic compound with the formula Et2NNO (Et = C2H5). A member of the nitrosamines, it is a light-sensitive, volatile, clear yellow oil that is soluble in water, lipids, and other organic solvents. It has an amine or aromatic odor. It is used as gasoline and lubricant additive, antioxidant, and stabilizer for industry materials. When heated to decomposition, N-nitrosodiethylamine emits toxic fumes of nitrogen oxides. N-Nitrosodiethylamine affects DNA integrity, probably by alkylation, and is used in experimental research to induce liver tumorigenesis. It is carcinogenic and mutagenic. NDEA has also been found to perturb amino acid biosynthesis including arginine, as well as DNA damage repair and mitochondrial genome maintenance in yeast.

Lupin Limited is an Indian multinational pharmaceutical company based in Mumbai. It is one of the largest generic pharmaceutical companies by revenue globally. The company's key focus areas include paediatrics, cardiovascular, anti-infectives, diabetology, asthma and anti-tuberculosis.
Unichem Laboratories is an Indian pharmaceutical company, headquartered in Mumbai. It manufactures and markets pharmaceutical formulations across the globe, including the regulated markets of the USA and Europe.

Intas Pharmaceuticals Limited is an Indian multinational pharmaceutical company headquartered in Ahmedabad. It is a producer of generic therapeutic drugs and engaged in contract clinical research and manufacturing. It has 22 manufacturing plants, 17 in India and the rest in Greece, United Kingdom and Mexico. In the financial year 2019, 69% of the company's revenue came from international markets while 31% came from India. It's market presence is more than 100+ countries.

Aurobindo Pharma Limited is an Indian multinational pharmaceutical manufacturing company headquartered in HITEC City, Hyderabad. The company manufactures generic pharmaceuticals and active pharmaceutical ingredients. The company's area of activity includes six major therapeutic and product areas: antibiotics, anti-retrovirals, cardiovascular products, central nervous system products, gastroenterologicals, and anti-allergics. The company markets these products in over 125 countries. Its marketing partners include AstraZeneca and Pfizer.

Taro Pharmaceutical Industries is an Israeli research-based pharmaceutical manufacturer publicly listed in the New York Stock Exchange. The company has more than 180 of its own drugs sold all over the world, reaching the markets of over 25 countries. The company's products are mainly sold in the United States, Canada and Israel. Taro Pharmaceutical Industries is reported to be on steady growth since 2008.

Cadila Pharmaceuticals is an Indian multinational pharmaceutical company based in Ahmedabad. The company's operations focus on manufacturing products ranging from active pharmaceutical intermediates, finished formulations, food supplements, biotechnology products and pharmaceutical machinery.
Alembic Pharmaceuticals Ltd. is an Indian multinational pharmaceutical company headquartered in Vadodara. It is involved in the manufacture of pharmaceutical products, pharmaceutical substances and intermediates. It is also termed to be a market leader in macrolides segment of anti-infective drugs in India.












