In biochemistry, a disulfide refers to a functional group with the structure R−S−S−R′. The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. Persulfide usually refers to R−S−S−H compounds.
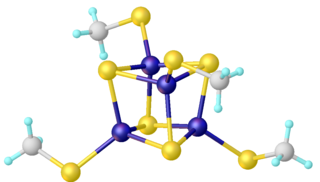
Iron–sulfur clusters are molecular ensembles of iron and sulfide. They are most often discussed in the context of the biological role for iron–sulfur proteins, which are pervasive. Many Fe–S clusters are known in the area of organometallic chemistry and as precursors to synthetic analogues of the biological clusters. It is believed that the last universal common ancestor had many iron-sulfur clusters.

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins.
Iron(III) chloride describes the inorganic compounds with the formula FeCl3(H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated forms which are both hygroscopic. They feature iron in its +3 oxidation state. The anhydrous derivative is a Lewis acid, while all forms are mild oxidizing agent. It is used as a water cleaner and as an etchant for metals.
Ferredoxins are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied to the "iron protein" first purified in 1962 by Mortenson, Valentine, and Carnahan from the anaerobic bacterium Clostridium pasteurianum.
Iron–sulfur proteins are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur clusters are found in a variety of metalloproteins, such as the ferredoxins, as well as NADH dehydrogenase, hydrogenases, coenzyme Q – cytochrome c reductase, succinate – coenzyme Q reductase and nitrogenase. Iron–sulfur clusters are best known for their role in the oxidation-reduction reactions of electron transport in mitochondria and chloroplasts. Both Complex I and Complex II of oxidative phosphorylation have multiple Fe–S clusters. They have many other functions including catalysis as illustrated by aconitase, generation of radicals as illustrated by SAM-dependent enzymes, and as sulfur donors in the biosynthesis of lipoic acid and biotin. Additionally, some Fe–S proteins regulate gene expression. Fe–S proteins are vulnerable to attack by biogenic nitric oxide, forming dinitrosyl iron complexes. In most Fe–S proteins, the terminal ligands on Fe are thiolate, but exceptions exist.

Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenols. An exception is the oxygen atom in the hydroxyl group (-OH) bonded to the aromatic ring is replaced by a sulfur atom. The prefix thio- implies a sulfur-containing compound and when used before a root word name for a compound which would normally contain an oxygen atom, in the case of 'thiol' that the alcohol oxygen atom is replaced by a sulfur atom.

Dithiolene metal complexes are complexes containing 1,2-dithiolene ligands. 1,2-Dithiolene ligands, a particular case of 1,2-dichalcogenolene species along with 1,2-diselenolene derivatives, are unsaturated bidentate ligand wherein the two donor atoms are sulfur. 1,2-Dithiolene metal complexes are often referred to as "metal dithiolenes", "metallodithiolenes" or "dithiolene complexes". Most molybdenum- and tungsten-containing proteins have dithiolene-like moieties at their active sites, which feature the so-called molybdopterin cofactor bound to the Mo or W.

Isopenicillin N synthase (IPNS) is a non-heme iron protein belonging to the 2-oxoglutarate (2OG)-dependent dioxygenases oxidoreductase family. This enzyme catalyzes the formation of isopenicillin N from δ-(L-α-aminoadipoyl)-L-cysteinyl-D-valine (LLD-ACV).
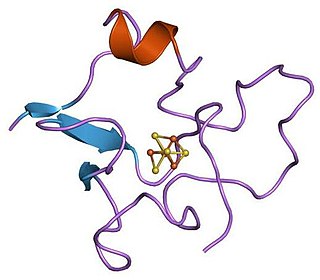
High potential iron-sulfur proteins (HIPIP) are a class of iron-sulfur proteins. They are ferredoxins that participate in electron transfer in photosynthetic bacteria as well as in Paracoccus denitrificans.

The Liebeskind–Srogl coupling reaction is an organic reaction forming a new carbon–carbon bond from a thioester and a boronic acid using a metal catalyst. It is a cross-coupling reaction. This reaction was invented by and named after Jiri Srogl from the Academy of Sciences, Czech Republic, and Lanny S. Liebeskind from Emory University, Atlanta, Georgia, USA. There are three generations of this reaction, with the first generation shown below. The original transformation used catalytic Pd(0), TFP = tris(2-furyl)phosphine as an additional ligand and stoichiometric CuTC = copper(I) thiophene-2-carboxylate as a co-metal catalyst. The overall reaction scheme is shown below.
In organometallic chemistry, bent metallocenes are a subset of metallocenes. In bent metallocenes, the ring systems coordinated to the metal are not parallel, but are tilted at an angle. A common example of a bent metallocene is Cp2TiCl2. Several reagents and much research is based on bent metallocenes.

Titanocene pentasulfide is the organotitanium compound with the formula (C5H5)2TiS5, commonly abbreviated as Cp2TiS5. This metallocene exists as a bright red solid that is soluble in organic solvents. It is of academic interest as a precursor to unusual allotropes of elemental sulfur as well as some related inorganic rings.
[NiFe] hydrogenase is a type of hydrogenase, which is an oxidative enzyme that reversibly converts molecular hydrogen in prokaryotes including Bacteria and Archaea. The catalytic site on the enzyme provides simple hydrogen-metabolizing microorganisms a redox mechanism by which to store and utilize energy via the reaction
In organometallic chemistry, a transition metal alkene complex is a coordination compound containing one or more alkene ligands. The inventory is large. Such compounds are intermediates in many catalytic reactions that convert alkenes to other organic products.

Acetyl-CoA synthase (ACS), not to be confused with acetyl-CoA synthetase or acetate-CoA ligase, is a nickel-containing enzyme involved in the metabolic processes of cells. Together with carbon monoxide dehydrogenase (CODH), it forms the bifunctional enzyme Acetyl-CoA Synthase/Carbon Monoxide Dehydrogenase (ACS/CODH) found in anaerobic organisms such as archaea and bacteria. The ACS/CODH enzyme works primarily through the Wood–Ljungdahl pathway which converts carbon dioxide to Acetyl-CoA. The recommended name for this enzyme is CO-methylating acetyl-CoA synthase.
Evolution of metal ions in biological systems refers to the incorporation of metallic ions into living organisms and how it has changed over time. Metal ions have been associated with biological systems for billions of years, but only in the last century have scientists began to truly appreciate the scale of their influence. Major and minor metal ions have become aligned with living organisms through the interplay of biogeochemical weathering and metabolic pathways involving the products of that weathering. The associated complexes have evolved over time.

Metal cluster compounds are a molecular ion or neutral compound composed of three or more metals and featuring significant metal-metal interactions.
Julia A. Kovacs is an American chemist specializing in bioinorganic chemistry. She is professor of chemistry at the University of Washington. Her research involves synthesizing small-molecule mimics of the active sites of metalloproteins, in order to investigate how cysteinates influence the function of non-heme iron enzymes, and the mechanism of the oxygen-evolving complex (OEC).

In organometallic chemistry, transition metal complexes of nitrite describes families of coordination complexes containing one or more nitrite ligands. Although the synthetic derivatives are only of scholarly interest, metal-nitrite complexes occur in several enzymes that participate in the nitrogen cycle.

![Structure of [Fe4S4(SMe)4] , a synthetic analogue of 4Fe-4S cofactors. OBINIX2.png](http://upload.wikimedia.org/wikipedia/commons/thumb/9/92/OBINIX2.png/220px-OBINIX2.png)


![Structure of the quasi-two-coordinate ferrous dithiolate Fe[SC6H3-2,6-(C6H2-2,4,6-( Pr)3)2]2. A weak Fe-C(ipso) bond is indicated by the Fe---C distance of 2.427(1) A. The structure illustrates the low coordination numbers enabled by bulky ligands. JARKAP.png](http://upload.wikimedia.org/wikipedia/commons/thumb/4/4a/JARKAP.png/220px-JARKAP.png)












