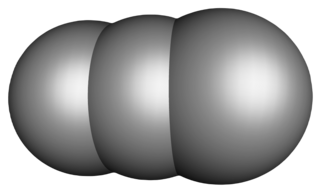Carbon monoxide is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest carbon oxide. In coordination complexes, the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry.

An oxide is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– ion with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 that protects the foil from further oxidation.

Sulfur monoxide is an inorganic compound with formula SO. It is only found as a dilute gas phase. When concentrated or condensed, it converts to S2O2 (disulfur dioxide). It has been detected in space but is rarely encountered intact otherwise.
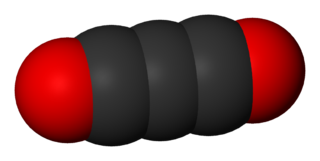
Carbon suboxide, or tricarbon dioxide, is an organic, oxygen-containing chemical compound with formula C3O2 and structure O=C=C=C=O. Its four cumulative double bonds make it a cumulene. It is one of the stable members of the series of linear oxocarbons O=Cn=O, which also includes carbon dioxide and pentacarbon dioxide. Although if carefully purified it can exist at room temperature in the dark without decomposing, it will polymerize under certain conditions.
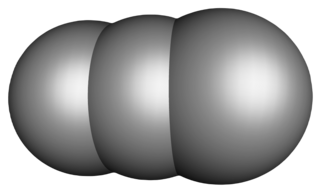
Tricarbon is an inorganic compound with the chemical formula C
2(μ-C). It is a colourless gas that only persists in dilution or solution as an adduct. It is one of the simplest unsaturated carbenes. Tricarbon can be found in interstellar space and can be produced in the laboratory by a process called laser ablation.

Dicarbon monoxide (C2O) is a molecule that contains two carbon atoms and one oxygen atom. It is a linear molecule that, because of its simplicity, is of interest in a variety of areas. It is, however, so extremely reactive that it is not encountered in everyday life. It is classified as a carbene, cumulene and an oxocarbon.
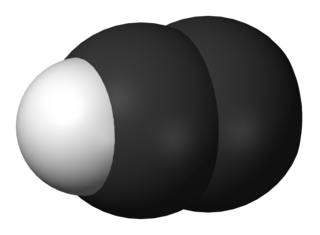
The ethynyl radical (systematically named λ3-ethyne and hydridodicarbon(C—C)) is an organic compound with the chemical formula C≡CH (also written [CCH] or C
2H). It is a simple molecule that does not occur naturally on Earth but is abundant in the interstellar medium. It was first observed by electron spin resonance isolated in a solid argon matrix at liquid helium temperatures in 1963 by Cochran and coworkers at the Johns Hopkins Applied Physics Laboratory. It was first observed in the gas phase by Tucker and coworkers in November 1973 toward the Orion Nebula, using the NRAO 11-meter radio telescope. It has since been detected in a large variety of interstellar environments, including dense molecular clouds, bok globules, star forming regions, the shells around carbon-rich evolved stars, and even in other galaxies.

Diatomic carbon (systematically named dicarbon and 1λ2,2λ2-ethene), is a green, gaseous inorganic chemical with the chemical formula C=C (also written [C2] or C2). It is kinetically unstable at ambient temperature and pressure, being removed through autopolymerisation. It occurs in carbon vapor, for example in electric arcs; in comets, stellar atmospheres, and the interstellar medium; and in blue hydrocarbon flames. Diatomic carbon is the second simplest of the allotropes of carbon (after atomic carbon), and is an intermediate participator in the genesis of fullerenes.
Formyl fluoride is the organic compound with the formula HC(O)F.

Atomic carbon, systematically named carbon and λ0-methane, is a colourless gaseous inorganic chemical with the chemical formula C. It is kinetically unstable at ambient temperature and pressure, being removed through autopolymerisation.

Silicon monoxide is the chemical compound with the formula SiO where silicon is present in the oxidation state +2. In the vapour phase, it is a diatomic molecule. It has been detected in stellar objects and has been described as the most common oxide of silicon in the universe.

In chemistry, an oxocarbon or oxide of carbon is a chemical compound consisting only of carbon and oxygen. The simplest and most common oxocarbons are carbon monoxide (CO) and carbon dioxide. Many other stable or metastable oxides of carbon are known, but they are rarely encountered, such as carbon suboxide and mellitic anhydride.
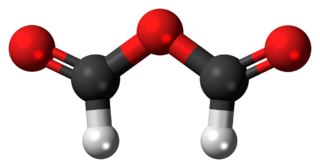
Formic anhydride, also called methanoic anhydride, is an organic compound with the chemical formula C
2H
2O
3 and a structural formula of (H(C=O)−)2O. It can be viewed as the anhydride of formic acid (HCOOH).

Disulfur monoxide or sulfur suboxide is an inorganic compound with the formula S2O, one of the lower sulfur oxides. It is a colourless gas and condenses to give a roughly dark red coloured solid that is unstable at room temperature.

Disulfur dioxide, dimeric sulfur monoxide or SO dimer is an oxide of sulfur with the formula S2O2. The solid is unstable with a lifetime of a few seconds at room temperature.
Polycarbonyl, is a solid, metastable, and explosive polymer of carbon monoxide. The polymer is produced by exposing carbon monoxide to high pressures. The structure of the solid appears amorphous, but may include a zig zag of equally-spaced CO groups.
A heterocumulene is a molecule or ion containing a chain of at least three double bonds between consecutive atoms, in which one or more atoms in the doubly bonded chain is a heteroatom. Such species are analogous to a cumulene in which the chain of doubly bonded atoms contains only carbon, except that at least one carbon is replaced by a heteroatom. Some authors relax the definition to include species with chains of only two double bonds between consecutive atoms, also known as heteroallenes.

Sulfoxylic acid (H2SO2) (also known as hyposulfurous acid or sulfur dihydroxide) is an unstable oxoacid of sulfur in an intermediate oxidation state between hydrogen sulfide and dithionous acid. It consists of two hydroxy groups attached to a sulfur atom. Sulfoxylic acid contains sulfur in an oxidation state of +2. Sulfur monoxide (SO) can be considered as a theoretical anhydride for sulfoxylic acid, but it is not actually known to react with water.
Tricarbon monosulfide (C3S) or tricarbon sulfur is a reactive molecular substance that has been detected in outer space. Tricarbon monosulfide is a heterocumulene or thiocumulene, consisting of a straight chain of three carbon atoms and a terminal sulfur atom.

1,1-Dimethyldiborane is the organoboron compound with the formula (CH3)2B(μ-H)2BH2. A pair of related 1,2-dimethyldiboranes are also known. It is a colorless gas that ignites in air.