Nitrogen fixation is a chemical process by which molecular nitrogen (N
2), which has a strong triple covalent bond, is converted into ammonia (NH
3) or related nitrogenous compounds, typically in soil or aquatic systems but also in industry. The nitrogen in air is molecular dinitrogen, a relatively nonreactive molecule that is metabolically useless to all but a few microorganisms. Biological nitrogen fixation or diazotrophy is an important microbe-mediated process that converts dinitrogen (N2) gas to ammonia (NH3) using the nitrogenase protein complex (Nif).

Cyanobacteria, also called Cyanobacteriota or Cyanophyta, are a phylum of autotrophic gram-negative bacteria that can obtain biological energy via photosynthesis. The name 'cyanobacteria' refers to their color, which similarly forms the basis of cyanobacteria's common name, blue-green algae, although they are not scientifically classified as algae. They appear to have originated in a freshwater or terrestrial environment.

Prochlorococcus is a genus of very small (0.6 μm) marine cyanobacteria with an unusual pigmentation. These bacteria belong to the photosynthetic picoplankton and are probably the most abundant photosynthetic organism on Earth. Prochlorococcus microbes are among the major primary producers in the ocean, responsible for a large percentage of the photosynthetic production of oxygen. Prochlorococcus strains, called ecotypes, have physiological differences enabling them to exploit different ecological niches. Analysis of the genome sequences of Prochlorococcus strains show that 1,273 genes are common to all strains, and the average genome size is about 2,000 genes. In contrast, eukaryotic algae have over 10,000 genes.

Trichodesmium, also called sea sawdust, is a genus of filamentous cyanobacteria. They are found in nutrient poor tropical and subtropical ocean waters. Trichodesmium is a diazotroph; that is, it fixes atmospheric nitrogen into ammonium, a nutrient used by other organisms. Trichodesmium is thought to fix nitrogen on such a scale that it accounts for almost half of the nitrogen fixation in marine systems globally. Trichodesmium is the only known diazotroph able to fix nitrogen in daylight under aerobic conditions without the use of heterocysts.
Diazotrophs are bacteria and archaea that fix atmospheric nitrogen(N2) in the atmosphere into bioavailable forms such as ammonia.
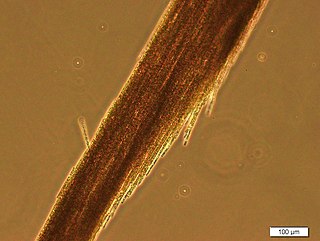
Aphanizomenon flos-aquae is a brackish and freshwater species of cyanobacteria of the genus Aphanizomenon found around the world, including the Baltic Sea and the Great Lakes.

Synechococcus is a unicellular cyanobacterium that is very widespread in the marine environment. Its size varies from 0.8 to 1.5 µm. The photosynthetic coccoid cells are preferentially found in well–lit surface waters where it can be very abundant. Many freshwater species of Synechococcus have also been described.

Cyanophages are viruses that infect cyanobacteria, also known as Cyanophyta or blue-green algae. Cyanobacteria are a phylum of bacteria that obtain their energy through the process of photosynthesis. Although cyanobacteria metabolize photoautotrophically like eukaryotic plants, they have prokaryotic cell structure. Cyanophages can be found in both freshwater and marine environments. Marine and freshwater cyanophages have icosahedral heads, which contain double-stranded DNA, attached to a tail by connector proteins. The size of the head and tail vary among species of cyanophages. Cyanophages infect a wide range of cyanobacteria and are key regulators of the cyanobacterial populations in aquatic environments, and may aid in the prevention of cyanobacterial blooms in freshwater and marine ecosystems. These blooms can pose a danger to humans and other animals, particularly in eutrophic freshwater lakes. Infection by these viruses is highly prevalent in cells belonging to Synechococcus spp. in marine environments, where up to 5% of cells belonging to marine cyanobacterial cells have been reported to contain mature phage particles.

Aphanizomenon is a genus of cyanobacteria that inhabits freshwater lakes and can cause dense blooms. They are unicellular organisms that consolidate into linear (non-branching) chains called trichomes. Parallel trichomes can then further unite into aggregates called rafts. Cyanobacteria such as Aphanizomenon are known for using photosynthesis to create energy and therefore use sunlight as their energy source. Aphanizomenon bacteria also play a big role in the Nitrogen cycle since they can perform nitrogen fixation. Studies on the species Aphanizomenon flos-aquae have shown that it can regulate buoyancy through light-induced changes in turgor pressure. It is also able to move by means of gliding, though the specific mechanism by which this is possible is not yet known.
Cyanobionts are cyanobacteria that live in symbiosis with a wide range of organisms such as terrestrial or aquatic plants; as well as, algal and fungal species. They can reside within extracellular or intracellular structures of the host. In order for a cyanobacterium to successfully form a symbiotic relationship, it must be able to exchange signals with the host, overcome defense mounted by the host, be capable of hormogonia formation, chemotaxis, heterocyst formation, as well as possess adequate resilience to reside in host tissue which may present extreme conditions, such as low oxygen levels, and/or acidic mucilage. The most well-known plant-associated cyanobionts belong to the genus Nostoc. With the ability to differentiate into several cell types that have various functions, members of the genus Nostoc have the morphological plasticity, flexibility and adaptability to adjust to a wide range of environmental conditions, contributing to its high capacity to form symbiotic relationships with other organisms. Several cyanobionts involved with fungi and marine organisms also belong to the genera Richelia, Calothrix, Synechocystis, Aphanocapsa and Anabaena, as well as the species Oscillatoria spongeliae. Although there are many documented symbioses between cyanobacteria and marine organisms, little is known about the nature of many of these symbioses. The possibility of discovering more novel symbiotic relationships is apparent from preliminary microscopic observations.
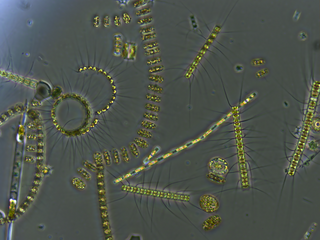
Bacterioplankton refers to the bacterial component of the plankton that drifts in the water column. The name comes from the Ancient Greek word πλανκτος, meaning "wanderer" or "drifter", and bacterium, a Latin term coined in the 19th century by Christian Gottfried Ehrenberg. They are found in both seawater and freshwater.
CandidatusAtelocyanobacterium thalassa, also referred to as UCYN-A, is a diazotrophic species of cyanobacteria commonly found in measurable quantities throughout the world's oceans and some seas. Members of A. thalassa are spheroid in shape and are 1-2 μm in diameter, and provide nitrogen to ocean regions by fixing non biologically available atmospheric nitrogen into biologically available ammonium that other marine microorganisms can use.
Anabaena variabilis is a species of filamentous cyanobacterium. This species of the genus Anabaena and the domain Eubacteria is capable of photosynthesis. This species is heterotrophic, meaning that it may grow without light in the presence of fructose. It also can convert atmospheric dinitrogen to ammonia via nitrogen fixation.
Raphidiopsis raciborskii is a freshwater cyanobacterium.

Cyanothece is a genus of unicellular, diazotrophic, oxygenic photosynthesizing cyanobacteria.

Crocosphaera watsonii is an isolate of a species of unicellular diazotrophic marine cyanobacteria which represent less than 0.1% of the marine microbial population. They thrive in offshore, open-ocean oligotrophic regions where the waters are warmer than 24 degrees Celsius. Crocosphaera watsonii cell density can exceed 1,000 cells per milliliter within the euphotic zone; however, their growth may be limited by the concentration of phosphorus. Crocosphaera watsonii are able to contribute to the oceanic carbon and nitrogen budgets in tropical oceans due to their size, abundance, and rapid growth rate. Crocosphaera watsonii are unicellular nitrogen fixers that fix atmospheric nitrogen to ammonia during the night and contribute to new nitrogen in the oceans. They are a major source of nitrogen to open-ocean systems. Nitrogen fixation is important in the oceans as it not only allows phytoplankton to continue growing when nitrogen and ammonium are in very low supply but it also replenishes other forms of nitrogen, thus fertilizing the ocean and allowing more phytoplankton growth.
Trichodesmium thiebautii is a cyanobacteria that is often found in open oceans of tropical and subtropical regions and is known to be a contributor to large oceanic surface blooms. This microbial species is a diazotroph, meaning it fixes nitrogen gas (N2), but it does so without the use of heterocysts. T. thiebautii is able to simultaneously perform oxygenic photosynthesis. T. thiebautii was discovered in 1892 by M.A. Gomont. T. thiebautii are important for nutrient cycling in marine habitats because of their ability to fix N2, a limiting nutrient in ocean ecosystems.

Oscillatoria brevis is a species of the genus Oscillatoria first identified in 1892. It is a blue-green filamentous cyanobacterium, which can be found in brackish and fresh waterways. O. brevis can also be isolated from soil.

Marine prokaryotes are marine bacteria and marine archaea. They are defined by their habitat as prokaryotes that live in marine environments, that is, in the saltwater of seas or oceans or the brackish water of coastal estuaries. All cellular life forms can be divided into prokaryotes and eukaryotes. Eukaryotes are organisms whose cells have a nucleus enclosed within membranes, whereas prokaryotes are the organisms that do not have a nucleus enclosed within a membrane. The three-domain system of classifying life adds another division: the prokaryotes are divided into two domains of life, the microscopic bacteria and the microscopic archaea, while everything else, the eukaryotes, become the third domain.
Richelia is a genus of nitrogen-fixing, filamentous, heterocystous and cyanobacteria. It contains the single species Richelia intracellularis. They exist as both free-living organisms as well as symbionts within potentially up to 13 diatoms distributed throughout the global ocean. As a symbiont, Richelia can associate epiphytically and as endosymbionts within the periplasmic space between the cell membrane and cell wall of diatoms.










