
Steric effects are nonbonding interactions that influence the shape (conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which usually dictate shape and reactivity. Steric effects result from repulsive forces between overlapping electron clouds.

In industrial chemistry, a stabilizer or stabiliser is a chemical that is used to prevent degradation. Heat and light stabilizers are added to plastics because they ensure safe processing and protect products against aging and weathering. The trend is towards fluid systems, pellets, and increased use of masterbatches. There are monofunctional, bifunctional, and polyfunctional stabilizers. In economic terms the most important product groups on the market for stabilizers are compounds based on calcium, lead, and tin stabilizers as well as liquid and light stabilizers. Cadmium-based stabilizers largely vanished in the last years due to health and environmental concerns.

Hydroperoxides or peroxols are compounds containing the hydroperoxide functional group (ROOH). If the R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. Organic hydroperoxides can either intentionally or unintentionally initiate explosive polymerisation in materials with unsaturated chemical bonds.

tert-Butyllithium is a chemical compound with the formula (CH3)3CLi. As an organolithium compound, it has applications in organic synthesis since it is a strong base, capable of deprotonating many carbon acids, including benzene. tert-Butyllithium is available commercially as hydrocarbon solutions; it is not usually prepared in the laboratory. Its synthesis was first reported by R. B. Woodward in 1941.

Di-tert-butyl dicarbonate is a reagent widely used in organic synthesis. Since this compound can be regarded formally as the acid anhydride derived from a tert-butoxycarbonyl (Boc) group, it is commonly referred to as Boc anhydride. This pyrocarbonate reacts with amines to give N-tert-butoxycarbonyl or so-called Boc derivatives. These carbamate derivatives do not behave as amines, which allows certain subsequent transformations to occur that would be incompatible with the amine functional group. The Boc group can later be removed from the amine using moderately strong acids. Thus, Boc serves as a protective group, for instance in solid phase peptide synthesis. Boc-protected amines are unreactive to most bases and nucleophiles, allowing for the use of the fluorenylmethyloxycarbonyl group (Fmoc) as an orthogonal protecting group.

2,4-Dimethyl-6-tert-butylphenol is the organic compound with the formula Me2(tert-Bu)C6H2OH (Me = methyl, tert-Bu = tertiary butyl). It is a colorless oil that is classified as an alkylated phenol.
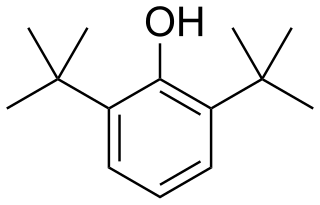
2,6-Di-tert-butylphenol is an organic compound with the structural formula 2,6-((CH3)3C)2C6H3OH. This colorless solid alkylated phenol and its derivatives are used industrially as UV stabilizers and antioxidants for hydrocarbon-based products ranging from petrochemicals to plastics. Illustrative of its usefulness, it prevents gumming in aviation fuels.
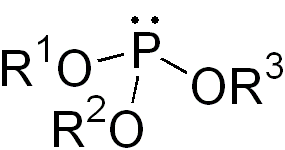
In chemistry a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of an unobserved tautomer phosphorous acid, H3PO3, with the simplest example being trimethylphosphite, P(OCH3)3. Some phosphites can be considered esters of the dominant tautomer of phosphorous acid (HP(O)(OH)2). The simplest representative is dimethylphosphite with the formula HP(O)(OCH3)2. Both classes of phosphites are usually colorless liquids.
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents.

The tert-butyloxycarbonyl protecting group or tert-butoxycarbonyl protecting group is a protecting group used in organic synthesis.

Di-tert-butylzinc is a compound with the formula ZnC8H18. This compound is used as a meta activating reagent in the syntheses of N,N-dimethyl-3-iodoaniline from N,N-dimethylaniline.
Monopotassium phosphite is an inorganic compound with the formula KH2PO3. A compositionally related compound has the formula H3PO3.2(KH2PO3). Both are white solids that consist of salts of the phosphite anion H2PO3−, the conjugate base of phosphorous acid. These materials have been used in some fertilizers.

2,6-Di-tert-butylpyridine is an organic compound with the formula (Me3C)2C5H3N. This colourless, oily liquid is derived from pyridine by replacement of the two H atoms with tert-butyl groups. It is a hindered base. For example, it can be protonated, but it does not form an adduct with boron trifluoride.
In organic chemistry, a tricarbonate is a compound containing the divalent [–O–(C=O)–O–(C=O)–O–(C=O)–O–] functional group, which consists of three carbonate groups in tandem, sharing two oxygen atoms. These compounds can be viewed as double esters of a hypothetical tricarbonic acid, HO–(C=O)–O–(C=O)–O–(C=O)–OH. An important example is di-tert-butyl tricarbonate (H3C–)3C–C3O7–C(–CH3)3, a chemical reagent (colorless prisms that melt at 62–63 °C with decomposition, soluble in pentane).
tert-Butyl bromide (also referred to as 2-bromo-2-methylpropane) is an organic compound with the formula Me3CBr (Me = methyl). The molecule features a tert-butyl group attached to a bromide substituent. This organobromine compound is used as a standard reagent in synthetic organic chemistry. It is a colorless liquid.
The Abramov reaction is the related conversions of trialkyl to α-hydroxy phosphonates by the addition to carbonyl compounds. In terms of mechanism, the reaction involves attack of the nucleophilic phosphorus atom on the carbonyl carbon. It was named after the Russian chemist Vasilii Semenovich Abramov (1904–1968) in 1957.

Diethyl chlorophosphate is an organophosphorus compound with the formula (C2H5O)2P(O)Cl. As an reagent in organic synthesis, it is use the convert alcohols to the corresponding diethylphosphate esters. It is a colorless liquid with a fruity odor. It is a corrosive, and as a cholinesterase inhibitor, highly toxic through dermal absorption. The molecule is tetrahedral.

Diethylphosphite is the organophosphorus compound with the formula (C2H5O)2P(O)H. It is a popular reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. Diethylphosphite is a colorless liquid. The molecule is tetrahedral.
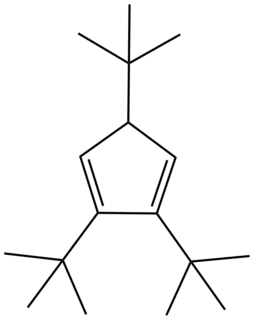
In the area of organometallic chemistry, a bulky cyclopentadienyl ligand is jargon for a ligand of the type C
5H
5−nR−
n where R is a branched alkyl and n = 3 or 4. Representative examples are the tetraisopropyl derivative C
5HiPr−
4 and the tris(tert-butyl) derivative 1,2,4-C
5H
2tBu−
3. These ligands are so large that their complexes behave differently from the pentamethylcyclopentadienyl analogues. Because they cannot closely approach the metal, these bulky ligands stabilize high spin complexes, such as (C5H2tBu3)2Fe2I2. These large ligands stabilize highly unsaturated derivatives such as (C5H2tBu3)2Fe2N2.

Di-tert-butylcyclopentadiene is an organic compound with the formula (Me3C)2C5H4, where Me = methyl. It is a colorless liquid that is soluble in organic solvents. The compound is the conjugate acid of the di-tert-butylcyclopentadienyl ligand, (Me
3C)
2C
5H−
3 (sometimes abbreviated Cp‡). Two regioisomers of di-tert-butylcyclopentadiene exist, depending on the location of the diene.















