The actinide or actinoid series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.
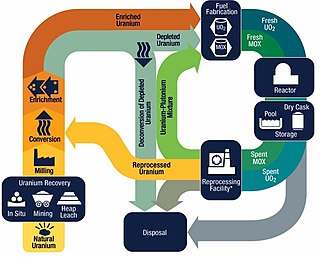
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in the back end, which are necessary to safely manage, contain, and either reprocess or dispose of spent nuclear fuel. If spent fuel is not reprocessed, the fuel cycle is referred to as an open fuel cycle ; if the spent fuel is reprocessed, it is referred to as a closed fuel cycle.
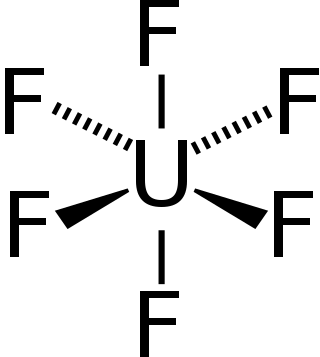
Uranium hexafluoride, sometimes called hex, is an inorganic compound with the formula UF6. Uranium hexafluoride is a volatile and toxic white solid that reacts with water, releasing corrosive hydrofluoric acid. The compound reacts mildly with aluminium, forming a thin surface layer of AlF3 that resists any further reaction from the compound. UF6 is used in the process of enriching uranium, which produces fuel for nuclear reactors and nuclear weapons.

Yellowcake is a type of uranium concentrate powder obtained from leach solutions, in an intermediate step in the processing of uranium ores. It is a step in the processing of uranium after it has been mined but before fuel fabrication or uranium enrichment. Yellowcake concentrates are prepared by various extraction and refining methods, depending on the types of ores. Typically, yellowcakes are obtained through the milling and chemical processing of uranium ore, forming a coarse powder that has a pungent odor, is insoluble in water, and contains about 80% uranium oxide, which melts at approximately 2880 °C.

Uranium oxide is an oxide of the element uranium.
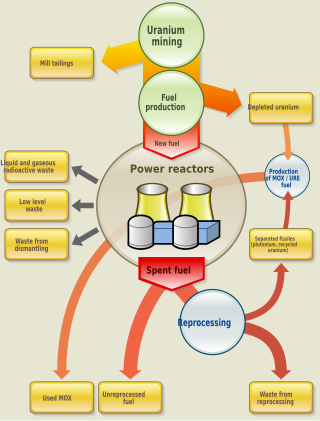
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
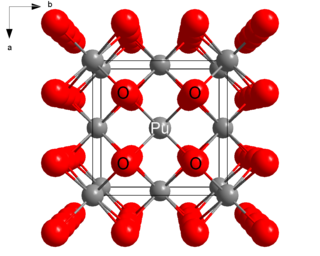
Plutonium(IV) oxide, or plutonia, is a chemical compound with the formula PuO2. This high melting-point solid is a principal compound of plutonium. It can vary in color from yellow to olive green, depending on the particle size, temperature and method of production.
Natural uranium is uranium with the same isotopic ratio as found in nature. It contains 0.711% uranium-235, 99.284% uranium-238, and a trace of uranium-234 by weight (0.0055%). Approximately 2.2% of its radioactivity comes from uranium-235, 48.6% from uranium-238, and 49.2% from uranium-234.

Uranium dioxide or uranium(IV) oxide , also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reactors. A mixture of uranium and plutonium dioxides is used as MOX fuel. Prior to 1960, it was used as yellow and black color in ceramic glazes and glass.
DUCRETE is a high density concrete alternative investigated for use in construction of casks for storage of radioactive waste. It is a composite material containing depleted uranium dioxide aggregate instead of conventional gravel, with a Portland cement binder.

The uranyl ion is an oxycation of uranium in the oxidation state +6, with the chemical formula UO2+
2. It has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligands may be bound to the uranyl ion in an equatorial plane around the uranium atom. The uranyl ion forms many complexes, particularly with ligands that have oxygen donor atoms. Complexes of the uranyl ion are important in the extraction of uranium from its ores and in nuclear fuel reprocessing.

Uranium trioxide (UO3), also called uranyl oxide, uranium(VI) oxide, and uranic oxide, is the hexavalent oxide of uranium. The solid may be obtained by heating uranyl nitrate to 400 °C. Its most commonly encountered polymorph, γ-UO3, is a yellow-orange powder.

Uranyl peroxide or uranium peroxide hydrate (UO4·nH2O) is a pale-yellow, soluble peroxide of uranium. It is found to be present at one stage of the enriched uranium fuel cycle and in yellowcake prepared via the in situ leaching and resin ion exchange system. This compound, also expressed as UO3·(H2O2)·(H2O), is very similar to uranium trioxide hydrate UO3·nH2O. The dissolution behaviour of both compounds are very sensitive to the hydration state (n can vary between 0 and 4). One main characteristic of uranium peroxide is that it consists of small needles with an average AMAD of about 1.1 μm.

A uranate is a ternary oxide involving the element uranium in one of the oxidation states 4, 5 or 6. A typical chemical formula is MxUyOz, where M represents a cation. The uranium atom in uranates(VI) has two short collinear U–O bonds and either four or six more next nearest oxygen atoms. The structures are infinite lattice structures with the uranium atoms linked by bridging oxygen atoms.

Ammonium uranyl carbonate (UO2CO3·2(NH4)2CO3) is known in the uranium processing industry as AUC and is also called uranyl ammonium carbonate. This compound is important as a component in the conversion process of uranium hexafluoride (UF6) to uranium dioxide (UO2). The ammonium uranyl carbonate is combined with steam and hydrogen at 500–600 °C to yield UO2. In another process aqueous uranyl nitrate, known as uranyl nitrate liquor (UNL) is treated with ammonium bicarbonate to form ammonium uranyl carbonate as a solid precipitate. This is separated from the solution, dried with methanol and then calcinated with hydrogen directly to UO2 to obtain a sinterable grade powder. The ex-AUC uranium dioxide powder is free-flowing, relatively coarse (10 µ) and porous with specific surface area in the range of 5 m2/g and suitable for direct pelletisation, avoiding the granulation step. Conversion to UO2 is often performed as the first stage of nuclear fuel fabrication.
Uranium compounds are compounds formed by the element uranium (U). Although uranium is a radioactive actinide, its compounds are well studied due to its long half-life and its applications. It usually forms in the +4 and +6 oxidation states, although it can also form in other oxidation states.
Paulscherrerite, UO2(OH)2, is a newly named mineral of the schoepite subgroup of hexavalent uranium hydrate/hydroxides. It is monoclinic, but no space group has been determined because no single-crystal study has been done. Paulscherrerite occurs as a canary yellow microcrystalline powdery product with a length of ~500 nm. It forms by the weathering and ultimate pseudomorphism of uranium-lead bearing minerals such as metaschoepite. The type locality for paulscherrerite is the Number 2 Workings, Radium Ridge near Mount Painter, North Flinders Ranges, South Australia, an area where radiogenic heat has driven hydrothermal activity for millions of years. It is named for Swiss physicist Paul Scherrer, co-inventor of the Debye-Scherrer X-ray powder diffraction camera. Study of paulscherrerite and related minerals is important for understanding the mobility of uranium around mining sites, as well as designing successful strategies for the storage of nuclear weapons and the containment of nuclear waste.

Actinide chemistry is one of the main branches of nuclear chemistry that investigates the processes and molecular systems of the actinides. The actinides derive their name from the group 3 element actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide. All but one of the actinides are f-block elements, corresponding to the filling of the 5f electron shell; lawrencium, a d-block element, is also generally considered an actinide. In comparison with the lanthanides, also mostly f-block elements, the actinides show much more variable valence. The actinide series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.

Uranium acid mine drainage refers to acidic water released from a uranium mining site using processes like underground mining and in-situ leaching. Underground, the ores are not as reactive due to isolation from atmospheric oxygen and water. When uranium ores are mined, the ores are crushed into a powdery substance, thus increasing surface area to easily extract uranium. The ores, along with nearby rocks, may also contain sulfides. Once exposed to the atmosphere, the powdered tailings react with atmospheric oxygen and water. After uranium extraction, sulfide minerals in uranium tailings facilitates the release of uranium radionuclides into the environment, which can undergo further radioactive decay while lowering the pH of a solution.
Meyrowitzite, Ca(UO2)(CO3)2·5H2O, is a carbonate mineral verified in May of 2018 by the Commission of New Minerals, Nomenclature and Classification of the International Mineralogical Association. It is an extremely rare mineral, discovered in the Markey mine Utah, U.S.A. The mineral is a transparent yellow and has blades up to approximately 0.2 mm in length. It is soluble in water or aqueous solutions. Meyrowitzite is named in honor of Robert Meyrowitz (1916–2013), an American analytical chemist. After serving in WW II, he joined the United States Geological Survey (USGS). He was known for developing innovative new methods for analyzing small and difficult to study mineralogical samples along with his formulation of the high-index immersion liquids.

















