
Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is a volatile liquid. Upon contact with humid air, it forms thick clouds of titanium dioxide and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines. It is sometimes referred to as “tickle” or “tickle 4”, as a phonetic representation of the symbols of its molecular formula.

Hafnium(IV) chloride is the inorganic compound with the formula HfCl4. This colourless solid is the precursor to most hafnium organometallic compounds. It has a variety of highly specialized applications, mainly in materials science and as a catalyst.
Boron trichloride is the inorganic compound with the formula BCl3. This colorless gas is a reagent in organic synthesis. It is highly reactive toward water.

Vanadium oxytrichloride is the inorganic compound with the formula VOCl3. This yellow distillable liquid hydrolyzes readily in air. It is an oxidizing agent. It is used as a reagent in organic synthesis. Samples often appear red or orange owing to an impurity of vanadium tetrachloride.

Zirconium(IV) chloride, also known as zirconium tetrachloride, is an inorganic compound frequently used as a precursor to other compounds of zirconium. This white high-melting solid hydrolyzes rapidly in humid air.

Tungsten hexachloride is an inorganic chemical compound of tungsten and chlorine with the chemical formula WCl6. This dark violet blue compound exists as volatile crystals under standard conditions. It is an important starting reagent in the preparation of tungsten compounds. Other examples of charge-neutral hexachlorides are rhenium(VI) chloride and molybdenum(VI) chloride. The highly volatile tungsten hexafluoride is also known.

Titanium tetraiodide is an inorganic compound with the formula TiI4. It is a black volatile solid, first reported by Rudolph Weber in 1863. It is an intermediate in the van Arkel–de Boer process for the purification of titanium.

Tungsten dichloride dioxide, or Tungstyl chloride is the chemical compound with the formula WO2Cl2. It is a yellow-colored solid. It is used as a precursor to other tungsten compounds. Like other tungsten halides, WO2Cl2 is sensitive to moisture, undergoing hydrolysis.
Tungsten(VI) oxytetrabromide is the inorganic compound with the formula WOBr4. This a red-brown, hygroscopic solid sublimes at elevated temperatures. It forms adducts with Lewis bases. The solid consists of weakly associated square pyramidal monomers. The related tungsten(VI) oxytetrachloride has been more heavily studied. The compound is usually classified as an oxyhalide.

Tungsten oxytetrafluoride is an inorganic compound with the formula WOF4. It is a colorless diamagnetic solid. The compound is one of many oxides of tungsten. It is usually encountered as product of the partial hydrolysis of tungsten hexafluoride.
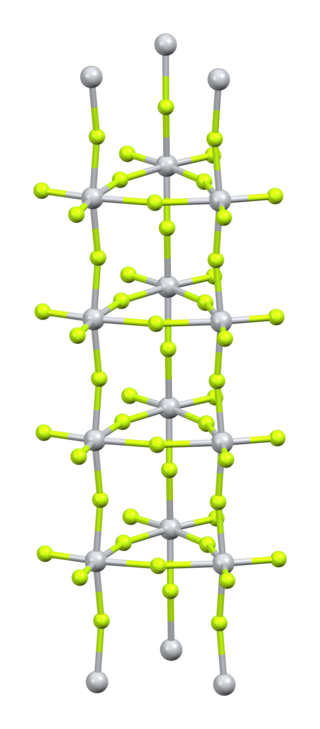
Titanium(IV) fluoride is the inorganic compound with the formula TiF4. It is a white hygroscopic solid. In contrast to the other tetrahalides of titanium, it adopts a polymeric structure. In common with the other tetrahalides, TiF4 is a strong Lewis acid.
Osmium compounds are compounds containing the element osmium (Os). Osmium forms compounds with oxidation states ranging from −2 to +8. The most common oxidation states are +2, +3, +4, and +8. The +8 oxidation state is notable for being the highest attained by any chemical element aside from iridium's +9 and is encountered only in xenon, ruthenium, hassium, iridium, and plutonium. The oxidation states −1 and −2 represented by the two reactive compounds Na
2[Os
4(CO)
13] and Na
2[Os(CO)
4] are used in the synthesis of osmium cluster compounds.
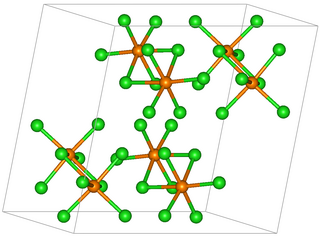
Rhenium pentachloride is an inorganic compound of chlorine and rhenium. The compound has the formula Re2Cl10 but it is usually referred to as rhenium pentachloride. It is a red-brown solid.

Molybdenum tetrachloride is the inorganic compound with the empirical formula MoCl4. The material exists as two polymorphs, both being dark-colored paramagnetic solids. These compounds are mainly of interest as precursors to other molybdenum complexes.

Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common.

Tris(trimethylsilyl)amine is the simplest tris(trialkylsilyl)amine which are having the general formula (R3Si)3N, in which all three hydrogen atoms of the ammonia are replaced by trimethylsilyl groups (-Si(CH3)3). Tris(trimethylsilyl)amine has been for years in the center of scientific interest as a stable intermediate in chemical nitrogen fixation (i. e. the conversion of atmospheric nitrogen N2 into organic substrates under normal conditions).

Molybdenum oxytetrachloride is the inorganic compound with the formula MoOCl4. This thermally unstable, dark green solid is used to prepare other complexes of molybdenum. It adopts a square pyramidal structure of C4v symmetry. As for other Mo(VI) compounds, it is diamagnetic. It decomposes thermally to MoOCl3.
Osmium tetrabromide is the inorganic compound with the formula OsBr4. A black solid, this compound can be produced by heating osmium tetrachloride and bromine under pressure.

In chemistry, a transition metal ether complex is a coordination complex consisting of a transition metal bonded to one or more ether ligand. The inventory of complexes is extensive. Common ether ligands are diethyl ether and tetrahydrofuran. Common chelating ether ligands include the glymes, dimethoxyethane (dme) and diglyme, and the crown ethers. Being lipophilic, metal-ether complexes often exhibit solubility in organic solvents, a property of interest in synthetic chemistry. In contrast, the di-ether 1,4-dioxane is generally a bridging ligand.
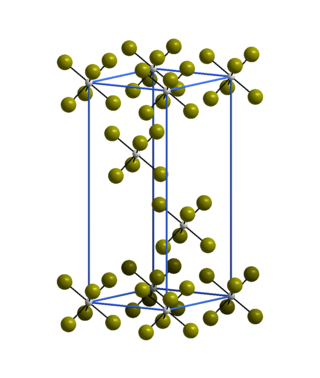
Tungsten hexabromide, also known as tungsten(VI) bromide, is a chemical compound of tungsten and bromine with the formula WBr6. It is an air-sensitive dark grey powder that decomposes above 200 °C to tungsten(V) bromide and bromine.


![Structure of solid WOCl4, illustrating its polymeric structure with short W[?]O and weak W---O bonds in the chains. Color code: O = red. WOCl4crystal.png](http://upload.wikimedia.org/wikipedia/commons/thumb/9/9f/WOCl4crystal.png/250px-WOCl4crystal.png)














