Related Research Articles
Benedict's reagent is a chemical reagent and complex mixture of sodium carbonate, sodium citrate and copper(II) sulfate pentahydrate. It is often used in place of Fehling's solution to detect the presence of reducing sugars. The presence of other reducing substances also gives a positive result. Such tests that use this reagent are called the Benedict's tests. A positive test with Benedict's reagent is shown by a color change from clear blue to brick-red with a precipitate.

A reagent is a substance or compound added to a system to cause a chemical reaction, or added to test if a reaction occurs. The terms reactant and reagent are often used interchangeably—however, a reactant is more specifically a substance consumed in the course of a chemical reaction. Solvents, though involved in the reaction, are usually not called reactants. Similarly, catalysts are not consumed by the reaction, so they are not reactants. In biochemistry, especially in connection with enzyme-catalyzed reactions, the reactants are commonly called substrates.

Copper(II) nitrate, Cu(NO3)2, is an inorganic compound that forms a blue crystalline solid. Anhydrous copper nitrate forms deep blue-green crystals and sublimes in a vacuum at 150-200 °C. Copper nitrate also occurs as five different hydrates, the most common ones being the hemipentahydrate and trihydrate.

Nitro compounds are organic compounds that contain one or more nitro functional groups (−NO2). The nitro group is one of the most common explosophores (functional group that makes a compound explosive) used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature, being almost invariably produced by nitration reactions starting with nitric acid.
Coulometry determines the amount of matter transformed during an electrolysis reaction by measuring the amount of electricity consumed or produced. It can be used for precision measurements of charge, and the amperes even used to have a coulometric definition. However, today coulometry is mainly used for analytical applications. Coulometry is a group of techniques in analytical chemistry. It is named after Charles-Augustin de Coulomb.
Acetylide refers to chemical compounds with the chemical formulas MC≡CH and MC≡CM, where M is a metal. The term is used loosely and can refer to substituted acetylides having the general structure RC≡CM. Acetylides are reagents in organic synthesis. The calcium acetylide commonly called calcium carbide is a major compound of commerce.

Tollens' reagent (chemical formula Ag(NH3)2NO3) is a chemical reagent used to determine the presence of aldehydes and aromatic aldehyde functional groups along with some alpha-hydroxy ketones which can tautomerize into aldehydes. The reagent consists of a solution of silver nitrate, ammonia and some sodium hydroxide (to maintain a basic pH of the reagent solution). It was named after its discoverer, the German chemist Bernhard Tollens. A positive test with Tollens' reagent is indicated by the precipitation of elemental silver, often producing a characteristic "silver mirror" on the inner surface of the reaction vessel.
Devarda's alloy, is an alloy of aluminium (44% – 46%), copper (49% – 51%) and zinc (4% – 6%).
In chemistry, a chemical test is a qualitative or quantitative procedure designed to identify, quantify, or characterise a chemical compound or chemical group.

Silver perchlorate is the chemical compound with the formula AgClO4. This white solid forms a monohydrate and is mildly deliquescent. It is a useful source of the Ag+ ion, although the presence of perchlorate presents risks. It is used as a catalyst in organic chemistry.

Thermospray is a soft ionization source by which a solvent flow of liquid sample passes through a very thin heated column to become a spray of fine liquid droplets. As a form of atmospheric pressure ionization in mass spectrometry these droplets are then ionized via a low-current discharge electrode to create a solvent ion plasma. A repeller then directs these charged particles through the skimmer and acceleration region to introduce the aerosolized sample to a mass spectrometer. It is particularly useful in liquid chromatography-mass spectrometry (LC-MS).

Silver hexafluorophosphate, sometimes referred to "silver PF-6," is an inorganic compound with the chemical formula AgPF6.
Flow injection analysis (FIA) is an approach to chemical analysis. It is accomplished by injecting a plug of sample into a flowing carrier stream. The principle is similar to that of Segmented Flow Analysis (SFA) but no air is injected into the sample or reagent streams.

Barium ferrate is the chemical compound of formula BaFeO4. This is a rare compound containing iron in the +6 oxidation state. The ferrate(VI) ion has two unpaired electrons, making it paramagnetic. It is isostructural with BaSO4, and contains the tetrahedral [FeO4]2− anion.

Ambient ionization is a form of ionization in which ions are formed in an ion source outside the mass spectrometer without sample preparation or separation. Ions can be formed by extraction into charged electrospray droplets, thermally desorbed and ionized by chemical ionization, or laser desorbed or ablated and post-ionized before they enter the mass spectrometer.
Organosilver chemistry in chemistry is the study of organometallic compounds containing a carbon to silver chemical bond and the study of silver as catalyst in organic reactions. In the group 11 elements silver is the element below copper. The chemistries have much in common but organosilver catalysis is much less common (mostly academic study) than organocopper chemistry due both to the relatively high price of silver and to the poor thermal stability of organosilver compounds. The oxidation state for silver in organosilver compounds in exclusively +1 with the notable exception of Ag(III) in the trifluoromethyl silver anion Ag(CF3)4− because of the electron-withdrawing effect of the trifluoromethyl groups. Poor thermal stability is reflected in decomposition temperatures of AgMe (-50 °C) versus CuMe (-15 °C) and PhAg (74 °C) vs PhCu (100 °C).
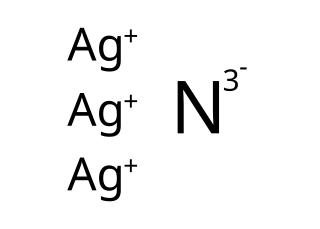
Silver nitride is an explosive chemical compound with symbol Ag3N. It is a black, metallic-looking solid which is formed when silver oxide or silver nitrate is dissolved in concentrated solutions of ammonia, causing formation of the diammine silver complex which subsequently breaks down to Ag3N. The standard free energy of the compound is about +315 kJ/mol, making it an endothermic compound which decomposes explosively to metallic silver and nitrogen gas.
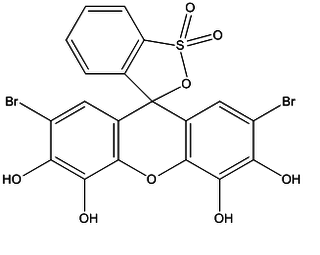
Bromopyrogallol red is frequently used in analytical chemistry as a reagent for spectrophometric analysis and as an complexometric indicator.

Desorption/ionization on silicon (DIOS) is a soft laser desorption method used to generate gas-phase ions for mass spectrometry analysis. DIOS is considered the first surface-based surface-assisted laser desorption/ionization (SALDI-MS) approach. Prior approaches were accomplished using nanoparticles in a matrix of glycerol, while DIOS is a matrix-free technique in which a sample is deposited on a nanostructured surface and the sample desorbed directly from the nanostructured surface through the adsorption of laser light energy. DIOS has been used to analyze organic molecules, metabolites, biomolecules and peptides, and, ultimately, to image tissues and cells.
Iodine nitrate is a chemical with formula INO3. (It differs from iodine nitrite with formula INO2.)
References
- 1 2 Mendham, J; Denney, R.C; Barnes, J.D.; Thomas, M. (2000). Vogel's Textbook of Quantitative Chemical Analysis (6th ed.). Pearson Education Ltd. ISBN 0-582-22628-7.
- 1 2 3 4 Walden, George H.; Hammett, Louis P.; Edmonds, Sylvan M. (1934). "Phenathroline-Ferrous Ion. III. A Silver Reductor. The Direct Determination of Iron in the Presence of Vanadium". Journal of the American Chemical Society. 56 (2): 350. doi:10.1021/ja01317a023.
- 1 2 3 Smith, G. F.; Cagle, F. W. (1948). "Preparation of silver for use in Walden silver reductor". Analytical Chemistry. 20 (2): 183. doi:10.1021/ac60014a024.