Strobilurins are a group of natural products and their synthetic analogs. A number of strobilurins are used in agriculture as fungicides. They are part of the larger group of QoIs, which act to inhibit the respiratory chain at the level of Complex III.

Friedrich Karl Rudolf Bergius was a German chemist known for the Bergius process for producing synthetic fuel from coal, Nobel Prize in Chemistry in recognition of contributions to the invention and development of chemical high-pressure methods. Having worked with IG Farben during World War II, his citizenship came into question following the war, causing him to ultimately flee to Argentina, where he acted as adviser to the Ministry of Industry.

Wolfgang Xavier Franz Ritter von Kobell was a German mineralogist and writer of short stories and poems in Bavarian dialect.

Adolf Friedrich Johann Butenandt was a German biochemist. He was awarded the Nobel Prize in Chemistry in 1939 for his "work on sex hormones." He initially rejected the award in accordance with government policy, but accepted it in 1949 after World War II. He was President of the Max Planck Society from 1960 to 1972. He was also the first, in 1959, to discover the structure of the sex pheromone of silkworms, which he named bombykol.

Ernst Otto Fischer was a German chemist who won the Nobel Prize for pioneering work in the area of organometallic chemistry.

Rudolf Criegee was a German organic chemist.
The Baeyer–Drewsen indigo synthesis (1882) is an organic reaction in which indigo is prepared from 2-nitrobenzaldehyde and acetone The reaction was developed by von Baeyer in 1880 to produce the first synthetic indigo at laboratory scale. This procedure is not used at industrial scale.
The Dakin–West reaction is a chemical reaction that transforms an amino-acid into a keto-amide using an acid anhydride and a base, typically pyridine. It is named for Henry Drysdale Dakin (1880–1952) and Randolph West (1890–1949). In 2016 Schreiner and coworkers reported the first asymmetric variant of this reaction employing short oligopeptides as catalysts.

Azoxystrobin is a broad spectrum systemic fungicide widely used in agriculture to protect crops from fungal diseases. It was first marketed in 1996 using the brand name Amistar and by 1999 it had been registered in 48 countries on more than 50 crops. In the year 2000 it was announced that it had been granted UK Millennium product status.

The Dacrymycetes are a class of fungi in the Basidiomycota. The class currently contains the single order Dacrymycetales, with a second proposed order Unilacrymales now treated at the family level. The order contains four families and has a cosmopolitan distribution.
Crell's Annalen is a German chemistry journal. Its original name is Chemische Annalen für die Freunde der Naturlehre, Arzneygelährtheit, Haushaltungskunst und Manufacturen, which is usually shortened to Chemische Annalen and often referred to as Crell's Annalen after the editor Lorenz Florenz Friedrich von Crell (1744–1816), professor of theoretical medicine and materia medica at the University of Helmstedt; it was first published in 1778.

Vulpinic acid is a natural product first found in and important in the symbiosis underlying the biology of lichens. It is a simple methyl ester derivative of its parent compound, pulvinic acid, and a close relative of pulvinone, both of which derive from aromatic amino acids such as phenylalanine via secondary metabolism. The roles of vulpinic acid are not fully established, but may include properties that make it an antifeedant for herbivores. The compound is relatively toxic to mammals.

Sinensetin is a methylated flavone. It can be found in Orthosiphon stamineus and in orange oil.

Heribert Offermanns is a German chemist and former member of the board of the Degussa AG.

Pulvinic acids are natural chemical pigments found in some lichens, derived biosynthetically from the aromatic amino acids phenylalanine and tyrosine, via dimerization and oxidative ring-cleavage of arylpyruvic acids, a process that also produces the related pulvinones.

Oudemansin A is a natural product first isolated from the basidiomycete fungus Oudemansiella mucida. Its chemical structure was determined by X-ray crystallography in 1979 and absolute stereochemistry by total synthesis. Two closely related derivatives, oudemansin B and X have also been isolated from other basidiomycetes. They are all biologically active against many filamentous fungi and yeasts but with insufficient potency and stability to become useful commercial products. However, their discovery, together with the strobilurins led to agricultural fungicides including azoxystrobin with the same mechanism of action.
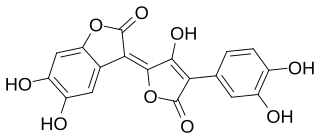
Variegatorubin is a pulvinic acid derivative. It is a red pigment that is present in many members of the Boletales, an order of the division Basidiomycota. It is generated from the oxidation of variegatic acid. Bolete species that contain variegatorubin include Neoboletus luridiformis, Chalciporus piperatus, Rhizopogon roseolus, Exsudoporus frostii, Suillellus luridus, Rubroboletus rhodoxanthus, and R. satanas. Variegatorubin was discovered by Wolfgang Steglich and colleagues, and described as a new compound in 1970.
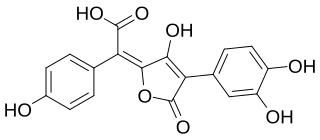
Xerocomic acid is a red-orange pigment found in fungi of the order Boletales. It is the precursor to variegatic acid, and is preceded by atromentic acid and atromentin. As an example, it is isolated from Serpula lacrymans. It is soluble in methanol. An oxidase acting on xerocomic acid is responsible for the "bluing" reaction seen in mushrooms.

Xerocomorubin is a pigment from the fungus order Boletales. It is the oxidized form of isoxerocomic acid. Air oxidation is responsible its formation, and it oxidizes faster to a similar pulvinic acid type pigment oxidized variant, variegatorubin. The long wavelength has an absorption at 497 nm, 106 nm higher than its precursor isoxerocomic acid. Synthesis experiments have shown tetra-acetylation by acetic anhydride and sulfuric acid. Although xerocomorubin and variegatorubin give off the same deep red color and could simultaneously occur in a mushroom, extracts from the deep red colored mushroom Boletus rubellus Krombh. identified only variegatorubin by thin layer chromatography (TLC), leading to the question the natural abundance of xerocomorubin.
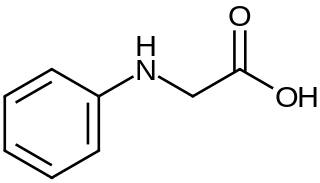
N-Phenylglycine is an organic compound with the formula C6H5NHCH2CO2H. This white solid achieved fame as the industrial precursor to indigo dye. It is a non-proteinogenic alpha amino acid related to sarcosine, but with an N-phenyl group in place of N-methyl.
















