Related Research Articles
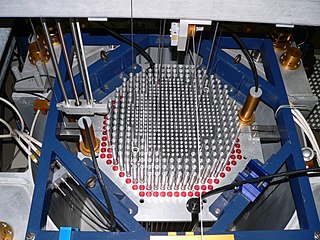
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nuclear fission is passed to a working fluid, which in turn runs through steam turbines. These either drive a ship's propellers or turn electrical generators' shafts. Nuclear generated steam in principle can be used for industrial process heat or for district heating. Some reactors are used to produce isotopes for medical and industrial use, or for production of weapons-grade plutonium. As of 2022, the International Atomic Energy Agency reports there are 422 nuclear power reactors and 223 nuclear research reactors in operation around the world.

Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the formation of xenon hexafluoroplatinate, the first noble gas compound to be synthesized.

A natural nuclear fission reactor is a uranium deposit where self-sustaining nuclear chain reactions occur. The conditions under which a natural nuclear reactor could exist were predicted in 1956 by Paul Kuroda. The remnants of an extinct or fossil nuclear fission reactor, where self-sustaining nuclear reactions have occurred in the past, are verified by analysis of isotope ratios of uranium and of the fission products. This was first discovered in 1972 in Oklo, Gabon by researchers from French Commissariat à l'énergie atomique (CEA) under conditions very similar to Kuroda's predictions.

Control rods are used in nuclear reactors to control the rate of fission of the nuclear fuel – uranium or plutonium. Their compositions include chemical elements such as boron, cadmium, silver, hafnium, or indium, that are capable of absorbing many neutrons without themselves decaying. These elements have different neutron capture cross sections for neutrons of various energies. Boiling water reactors (BWR), pressurized water reactors (PWR), and heavy-water reactors (HWR) operate with thermal neutrons, while breeder reactors operate with fast neutrons. Each reactor design can use different control rod materials based on the energy spectrum of its neutrons. Control rods have been used in nuclear aircraft engines like Project Pluto as a method of control.

Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release of heat energy, and gamma rays. The two smaller nuclei are the fission products..
In nuclear engineering, the void coefficient is a number that can be used to estimate how much the reactivity of a nuclear reactor changes as voids form in the reactor moderator or coolant. Net reactivity in a reactor depends on several factors, one of which is the void coefficient. Reactors in which either the moderator or the coolant is a liquid will typically have a void coefficient which is either negative or positive. Reactors in which neither the moderator nor the coolant is a liquid will have a zero void coefficient. It is unclear how the definition of "void" coefficient applies to reactors in which the moderator/coolant is neither liquid nor gas.
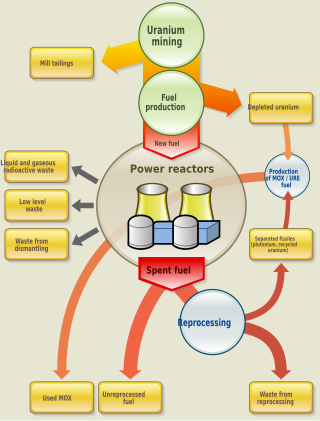
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission. Nuclear fuel has the highest energy density of all practical fuel sources. The processes involved in mining, refining, purifying, using, and disposing of nuclear fuel are collectively known as the nuclear fuel cycle.
Naturally occurring samarium (62Sm) is composed of five stable isotopes, 144Sm, 149Sm, 150Sm, 152Sm and 154Sm, and two extremely long-lived radioisotopes, 147Sm (half life: 1.06×1011 y) and 148Sm (6.3×1015 y), with 152Sm being the most abundant (26.75% natural abundance). 146Sm is also fairly long-lived, but is not long-lived enough to have survived in significant quantities from the formation of the Solar System on Earth, although it remains useful in radiometric dating in the Solar System as an extinct radionuclide. A 2012 paper revising the estimated half-life of 146Sm from 10.3(5)×107 y to 6.8(7)×107 y was retracted in 2023. It is the longest-lived nuclide that has not yet been confirmed to be primordial.
Naturally occurring xenon (54Xe) consists of seven stable isotopes and two very long-lived isotopes. Double electron capture has been observed in 124Xe and double beta decay in 136Xe, which are among the longest measured half-lives of all nuclides. The isotopes 126Xe and 134Xe are also predicted to undergo double beta decay, but this process has never been observed in these isotopes, so they are considered to be stable. Beyond these stable forms, 32 artificial unstable isotopes and various isomers have been studied, the longest-lived of which is 127Xe with a half-life of 36.345 days. All other isotopes have half-lives less than 12 days, most less than 20 hours. The shortest-lived isotope, 108Xe, has a half-life of 58 μs, and is the heaviest known nuclide with equal numbers of protons and neutrons. Of known isomers, the longest-lived is 131mXe with a half-life of 11.934 days. 129Xe is produced by beta decay of 129I ; 131mXe, 133Xe, 133mXe, and 135Xe are some of the fission products of both 235U and 239Pu, so are used as indicators of nuclear explosions.

There are 37 known isotopes of iodine (53I) from 108I to 144I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element.
Caesium (55Cs) has 41 known isotopes, the atomic masses of these isotopes range from 112 to 152. Only one isotope, 133Cs, is stable. The longest-lived radioisotopes are 135Cs with a half-life of 1.33 million years, 137
Cs
with a half-life of 30.1671 years and 134Cs with a half-life of 2.0652 years. All other isotopes have half-lives less than 2 weeks, most under an hour.
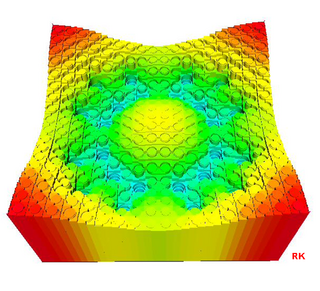
Nuclear reactor physics is the field of physics that studies and deals with the applied study and engineering applications of chain reaction to induce a controlled rate of fission in a nuclear reactor for the production of energy. Most nuclear reactors use a chain reaction to induce a controlled rate of nuclear fission in fissile material, releasing both energy and free neutrons. A reactor consists of an assembly of nuclear fuel, usually surrounded by a neutron moderator such as regular water, heavy water, graphite, or zirconium hydride, and fitted with mechanisms such as control rods which control the rate of the reaction.
In applications such as nuclear reactors, a neutron poison is a substance with a large neutron absorption cross-section. In such applications, absorbing neutrons is normally an undesirable effect. However, neutron-absorbing materials, also called poisons, are intentionally inserted into some types of reactors in order to lower the high reactivity of their initial fresh fuel load. Some of these poisons deplete as they absorb neutrons during reactor operation, while others remain relatively constant.
Iodine-125 (125I) is a radioisotope of iodine which has uses in biological assays, nuclear medicine imaging and in radiation therapy as brachytherapy to treat a number of conditions, including prostate cancer, uveal melanomas, and brain tumors. It is the second longest-lived radioisotope of iodine, after iodine-129.

Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor. It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and, depending on its point along the nuclear fuel cycle, it will have different isotopic constituents than when it started.

This page discusses each of the main elements in the mixture of fission products produced by nuclear fission of the common nuclear fuels uranium and plutonium. The isotopes are listed by element, in order by atomic number.
Uranium-236 (236U) is an isotope of uranium that is neither fissile with thermal neutrons, nor very good fertile material, but is generally considered a nuisance and long-lived radioactive waste. It is found in spent nuclear fuel and in the reprocessed uranium made from spent nuclear fuel.
Nuclear fission splits a heavy nucleus such as uranium or plutonium into two lighter nuclei, which are called fission products. Yield refers to the fraction of a fission product produced per fission.
Long-lived fission products (LLFPs) are radioactive materials with a long half-life produced by nuclear fission of uranium and plutonium. Because of their persistent radiotoxicity, it is necessary to isolate them from humans and the biosphere and to confine them in nuclear waste repositories for geological periods of time. The focus of this article is radioisotopes (radionuclides) generated by fission reactors.
The iodine pit, also called the iodine hole or xenon pit, is a temporary disabling of a nuclear reactor due to buildup of short-lived nuclear poisons in the reactor core. The main isotope responsible is 135Xe, mainly produced by natural decay of 135I. 135I is a weak neutron absorber, while 135Xe is the strongest known neutron absorber. When 135Xe builds up in the fuel rods of a reactor, it significantly lowers their reactivity, by absorbing a significant amount of the neutrons that provide the nuclear reaction.
References
- 1 2 "Livechart - Table of Nuclides - Nuclear structure and decay data".
- ↑ ""Xenon Poisoning" or Neutron Absorption in Reactors".
- ↑ Benczer-Koller, Noemie (January 2009). "Chien-shiungwu 1912—1997" (PDF).
- ↑ Lykknes, Annette (2019-01-02). Women In Their Element: Selected Women's Contributions To The Periodic System. World Scientific. ISBN 9789811206306.
- ↑ DOE Fundamentals Handbook: Nuclear Physics and Reactor Theory Volume 2 (PDF). U.S. Department of Energy. January 1993. Archived from the original (PDF) on 2013-02-14., pp. 35–42.
- ↑ DOE Fundamentals Handbook: Nuclear Physics and Reactor Theory Volume 2 (PDF). U.S. Department of Energy. January 1993. Archived from the original (PDF) on 2013-02-14., p. 35.
- ↑ Crist, J. E. "Xenon, A Fission Product Poison" (PDF). candu.org. Archived from the original (PDF) on February 3, 2007. Retrieved 2 November 2011.
- ↑ Xenon decay transient graph Archived June 24, 2018, at the Wayback Machine
- ↑ CANDU Fundamentals: 20 Xenon: A Fission Product Poison Archived July 23, 2011, at the Wayback Machine
- ↑ Utilization of the Isotopic Composition of Xe and Kr in Fission Gas Release Research Archived October 19, 2013, at the Wayback Machine
- ↑ Roggenkamp, Paul L. "The Influence of Xenon-135 on Reactor Operation" (PDF). Westinghouse Savannah River Company. Retrieved 18 October 2013.
- ↑ "Xenon-135". www.nuclear-power.net. Retrieved 2017-09-19. and "Xenon-135 Oscillations".